��Ŀ����
ijѧϰС�鰴��ͼ��ʵ������ȡ������̽��ͭ���й����ʣ����ּг�����δ��������
��ش�
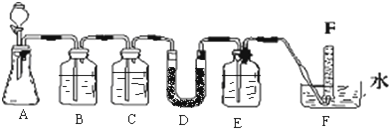
��1����ȡ�����Ļ�ѧ����ʽ��______��
��2����ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ______���ڼ�ʯ�ҵ�������______��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
��ʵ����Ŀ����______��
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ��______��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ��______��

��ش�
��1����ȡ�����Ļ�ѧ����ʽ��______��
��2����ʵ������Ϊ����ɫCuO��Ϊ��ɫ�����ɵ���a������ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ______���ڼ�ʯ�ҵ�������______��
��3�����������ɵĵ���a����ˮԡ�н���4��ʵ�飬����ʵ�鱨�����±���ʾ��
| ��� | ʵ����� | ʵ������ |
| �� | ϡ�����м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ��������Һ�м���õ���a | �����Ա仯 |
| �� | ϡ�����м�����������Һ | �����Ա仯 |
| �ټ���õ���a | ����ɫ���ݣ���Һ���� |
��ʵ����з�Ӧ�ı����ǣ������ӷ���ʽ��ʾ��______��
�۽��õ���a���뵽��������Ĺ���������Һ�У���Һ�����ɫ���÷�Ӧ�����ӷ���ʽ��______��
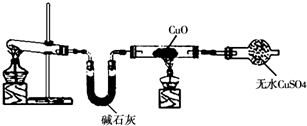
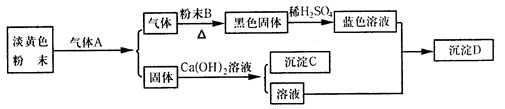
��1���Ȼ�狀��������Ʒ������ֽⷴӦ�������Ȼ��ƺͰ�����ˮ���ʴ�Ϊ��Ca��OH��2+2NH4Cl
CaCl2+2H2O+2NH3����
��2���ٸ���ʵ�������ж��������ʹ��ɫ��ˮ����ͭ��ĩ��Ϊ��ɫ��������ˮ����ɫ������ͭ��Ϊ��ɫ��������ͭ�����������ԭ����ͭ��������������ԭ��Ӧ�ã�����ɫ����Ⱦ�������ǵ������ʴ�Ϊ��3CuO+2NH3
3Cu+N2+3H2O��
�ڼ�ʯ�ҵijɷ��� CaO��NaOH�Ļ���CaO�ܺ�ˮ��Ӧ��NaOH�׳��⣬���������������
�ʴ�Ϊ�����հ����л��е�ˮ��������ֹ���Ų���ˮ�ļ�����
��3���ٸ���ʵ������֪�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ⣬����ʵ�����֤��ͭ�������ӡ������ӡ���������Ӻ���������Ӷ�����Ӧ��
�ʴ�Ϊ��˵�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ⣻
�����������£�ͭ����������ӷ���������ԭ��Ӧ����ͭ���ӡ�һ�����������ˮ�����ӷ�Ӧ����ʽΪ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O��
�����������£���ϡ�����м���ͭ����Һ����ɫ��˵��ͭ����������ͭ���ӣ���˫��ˮ����ԭ����ˮ�����ӷ���ʽΪ��Cu+2H++H2O2=Cu2++2H2O���ʴ�Ϊ��Cu+2H++H2O2=Cu2++2H2O��
| ||
��2���ٸ���ʵ�������ж��������ʹ��ɫ��ˮ����ͭ��ĩ��Ϊ��ɫ��������ˮ����ɫ������ͭ��Ϊ��ɫ��������ͭ�����������ԭ����ͭ��������������ԭ��Ӧ�ã�����ɫ����Ⱦ�������ǵ������ʴ�Ϊ��3CuO+2NH3
| ||
�ڼ�ʯ�ҵijɷ��� CaO��NaOH�Ļ���CaO�ܺ�ˮ��Ӧ��NaOH�׳��⣬���������������
�ʴ�Ϊ�����հ����л��е�ˮ��������ֹ���Ų���ˮ�ļ�����
��3���ٸ���ʵ������֪�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ⣬����ʵ�����֤��ͭ�������ӡ������ӡ���������Ӻ���������Ӷ�����Ӧ��
�ʴ�Ϊ��˵�������ӡ���������ӡ���������Ӿ����ܵ���ʹͭ�ܽ⣻
�����������£�ͭ����������ӷ���������ԭ��Ӧ����ͭ���ӡ�һ�����������ˮ�����ӷ�Ӧ����ʽΪ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O���ʴ�Ϊ��3Cu+2NO3-+8H+=3Cu2++2NO��+4H2O��
�����������£���ϡ�����м���ͭ����Һ����ɫ��˵��ͭ����������ͭ���ӣ���˫��ˮ����ԭ����ˮ�����ӷ���ʽΪ��Cu+2H++H2O2=Cu2++2H2O���ʴ�Ϊ��Cu+2H++H2O2=Cu2++2H2O��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 ��1�� ijѧ������֪����y�˵ı�����ȷ��ȡW����Ʒ������������ƽ�����̷��루W+y�������룬�����̵ı������м�����Ʒ����ʱָ��ƫ���ұߣ�����ͼʾ���벹��������IJ�����_______��
��1�� ijѧ������֪����y�˵ı�����ȷ��ȡW����Ʒ������������ƽ�����̷��루W+y�������룬�����̵ı������м�����Ʒ����ʱָ��ƫ���ұߣ�����ͼʾ���벹��������IJ�����_______��