��Ŀ����
����Ŀ����ͼ�ǽ���þ��±�ص���(X2)��Ӧ�������仯ʾ��ͼ������˵����ȷ����( )
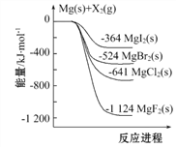
A. ����þ��±�ص���(X2)�ķ�Ӧ���Է���������Ϊ��H��С����
B. ���ȶ��ԣ�MgI2 >MgBr2 >MgCl2 >MgF2
C. ��ҵ�Ͽ��ɵ��MgCl2��Һұ������Mg���ù�������������
D. ��ͼ��֪���¶���MgBr2(s)��Cl2(g)��Ӧ���Ȼ�ѧ����ʽΪ��MgBr2(s)+Cl2(g)![]() MgCl2(s)+Br2��g����H=+117kJ��mol��1
MgCl2(s)+Br2��g����H=+117kJ��mol��1
���𰸡�A
��������
A����ͼ��֪����Ϊ��Ӧ��������������������������
B�������͵������ȶ���
C�����MgCl2��Һ����������þ��������������
D����ͼ��֪Mg��s��+Cl2��l��=MgCl2��s����H=-641kJ��mol��1��Mg��s��+Br2��l��=MgBr2��s����H=-524kJ��mol��1����ϸ�˹���������
A����ͼ��֪����Ϊ��Ӧ����������������������������Ϊ���ȷ�Ӧ�������þ��±�ص��ʣ�X2���ķ�Ӧ���Է���������Ϊ��H��С���㣬��A��ȷ��
B�������͵������ȶ�����������ȶ���˳��Ϊ��MgI2��MgBr2��MgCl2��MgF2����B����
C�����MgCl2��Һ����������þ�����������������ʱ����ת��Ϊ��ѧ�ܣ�������������C����
D����ͼ��֪Mg��s��+Cl2��l��=MgCl2��s����H=-641kJ��mol��1��Mg��s��+Br2��l��=MgBr2��s����H=-524kJ��mol��1����ϸ�˹���ɣ�����һ������ʽ��ȥ�ڶ�����ʽ��MgBr2��s��+Cl2��g���TMgCl2��s��+Br2��g����H=-117KJ��mol��1����D����
��ѡ��A��

����Ŀ��ij�о���ѧϰС���������ռ�����Ϣ���ơ�þ�Ȼ��ý���������CO2������ȼ�ա�
���Ƕ�����CO2������ȼ�պ�õ��İ�ɫ�������������̽����
[ʵ�����]��ȼ�յ���Ѹ������װ��CO2�ļ���ƿ�У��������м���ȼ�գ���Ӧ����ȴ��ƿ���к�ɫ������ƿ���ϸ��Ű�ɫ���ʡ�
[�������]����1����ɫ������Na2O��
����2����ɫ������Na2CO3��
����3����ɫ������Na2O��Na2CO3�Ļ���
[��Ʒ���]��С���ȼ�պ����ɵİ�ɫ���ʽ�������̽����
ʵ�鷽�� | ʵ����� | ʵ������ | ���� |
����1 | ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м�����ɫ��̪��Һ | ��Һ��ɺ�ɫ | ��ɫ����ΪNa2O |
����2 | ��ȡ������ɫ�������Թ��У���������ˮ������Ʒȫ������ˮ�������м��������CaCl2��Һ�� �ھ���Ƭ�̣�ȡ�ϲ���Һ���Թ��У��μ���ɫ��̪��Һ | �ٳ��ְ�ɫ���� ������������ | ��ɫ����ΪNa2CO3 |
��ش��������⣺
(1)д������þ�������̼��Ӧ�Ļ�ѧ����ʽ��________________________��
(2)��ͬѧ��Ϊ����1�õ��Ľ��۲���ȷ����������____________________��
(3)���ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ______________________________��
(4)��ͬѧ��Ϊ��ɫ�����п������������ơ����Ƿ�ͬ����ͬѧ�Ĺ۵㣬���������ɣ�__________��