��Ŀ����
����Ŀ����1��ij�л�����C��H��O����Ԫ����ɣ����ģ����ͼ��ʾ��
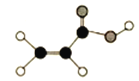
�ٺ��еĹ�����������_______��
��д�����л���������Ʒ�Ӧ�Ļ�ѧ����ʽ_______��
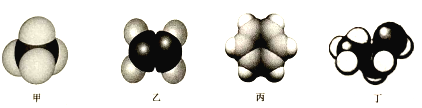
��2�����ֳ����л�����ӵı���ģ��ʾ��ͼ���£����мס��ҡ���Ϊ������Ϊ���������
�ٿ��Լ�����ҵ��Լ�Ϊ_________��
a.ϡ���� b.������Ȼ�̼��Һ c.ˮ d.���Ը��������Һ
�������������ж�����������ζ���Ҳ�����ˮ���ܶȱ�ˮС����_________�������ƣ������������ˮ�У����ã��۲쵽��������_______��
���ҺͶ������ʵ�����1.5mol����ȫȼ����Ҫ�����������ʵ�����_______��
��3�����ᣨ![]() ����һ���л�����ڶ������ﻯѧ����������Ҫ���á�
����һ���л�����ڶ������ﻯѧ����������Ҫ���á�
��1mol������������Na��Ӧ�������������ʵ�����_______��
�����������۷�Ӧ������������������������ȱ����ƶѪ����Ӧ�Ļ�ѧ����ʽ��_______________��
���𰸡�̼̼˫�����Ȼ�2CH2=CH-COOH+2Na��2CH2=CH- COONa+H2��bd���ֲ㣬��ˮ����ɫ��ȥ���ϲ�ʳȺ�ɫ4.5mol1mol2![]() +Fe����CH3CH(OH)COO��2Fe+H2��
+Fe����CH3CH(OH)COO��2Fe+H2��
��������
��1�����ݼۼ���������ģ�ͣ����л���Ľṹ��ʽΪCH2=CHCOOH�����к��еĹ�����Ϊ̼̼˫�����Ȼ����Ȼ�����Na��Ӧ��
��2���ס��ҡ���Ϊ������Ϊ�����������ϱ���ģ�����Ľṹ��ʽΪCH4���ҵĽṹ��ʽΪCH2=CH2�����Ľṹ��ʽΪ![]() �����Ľṹ��ʽΪCH3CH2OH�������������ʵ���������
�����Ľṹ��ʽΪCH3CH2OH�������������ʵ���������
��3���������еĴ��ǻ����Ȼ�������Na��Ӧ��
���ǻ���Ļ���С���Ȼ���Ļ��ԣ��������Ȼ�����Fe��Ӧ��
��1�����ݼۼ���������ģ�ͣ����л���Ľṹ��ʽΪCH2=CHCOOH��
�����л����к��еĹ���������Ϊ̼̼˫�����Ȼ���
�����л����к��Ȼ�����Na��Ӧ����Ӧ�Ļ�ѧ����ʽΪ2CH2=CHCOOH+2Na��2CH2=CHCOONa+H2����
��2���ס��ҡ���Ϊ������Ϊ�����������ϱ���ģ�����Ľṹ��ʽΪCH4���ҵĽṹ��ʽΪCH2=CH2�����Ľṹ��ʽΪ![]() �����Ľṹ��ʽΪCH3CH2OH��
�����Ľṹ��ʽΪCH3CH2OH��
����Ϊ���飬��Ϊ��ϩ��a����CH4��CH2=CH2��ϡ���ᶼ����Ӧ������������������ϡ�������CH4��CH2=CH2��b����CH4����ʹ������Ȼ�̼��Һ��ɫ��CH2=CH2��������Ȼ�̼��Һ�����ӳɷ�Ӧ��CH2=CH2��ʹ������Ȼ�̼��Һ��ɫ������ͬ������������Ȼ�̼��Һ����CH4��CH2=CH2��c�CH4��CH2=CH2��ˮ������Ӧ������������������ˮ����CH4��CH2=CH2��d����CH4����ʹ����KMnO4��Һ��ɫ��CH2=CH2��ʹ����KMnO4��Һ��ɫ������ͬ����������KMnO4��Һ����CH4��CH2=CH2��������������ҵ��Լ�Ϊ������Ȼ�̼��Һ������KMnO4��Һ����ѡbd��
�����������������ж�����������ζ���Ҳ�����ˮ���ܶȱ�ˮС���DZ�������������ˮ�У����ã�����ȡ����ˮ�е�Br2���۲쵽�������ǣ��ֲ㣬��ˮ����ɫ��ȥ���ϲ�ʳȺ�ɫ��
���ҡ����ֱ�ΪCH2=CH2��CH3CH2OH��CH2=CH2��CH3CH2OH�ֱ���ȫȼ�յĻ�ѧ����ʽΪCH2=CH2+3O2![]() 2CO2+2H2O��CH3CH2OH+3O2
2CO2+2H2O��CH3CH2OH+3O2![]() 2CO2+3H2O���ɼ�1molCH2=CH2��1molCH3CH2OH��ȫȼ�ն�����3molO2����1.5molCH2=CH2��CH3CH2OH��ȫȼ����ҪO2���ʵ���Ϊ1.5mol
2CO2+3H2O���ɼ�1molCH2=CH2��1molCH3CH2OH��ȫȼ�ն�����3molO2����1.5molCH2=CH2��CH3CH2OH��ȫȼ����ҪO2���ʵ���Ϊ1.5mol![]() 3=4.5mol��
3=4.5mol��
��3���������к�1���ǻ���1���Ȼ����������ǻ����Ȼ�������Na��Ӧ����Ӧ�Ļ�ѧ����ʽΪ![]() +2Na��
+2Na��![]() +H2����1mol����������Na��Ӧ����H2���ʵ���Ϊ1mol��
+H2����1mol����������Na��Ӧ����H2���ʵ���Ϊ1mol��
���ǻ���Ļ���С���Ȼ���Ļ��ԣ��������Ȼ���Fe��Ӧ�����������۷�Ӧ�������������Ļ�ѧ����ʽΪ2![]() +Fe����
+Fe����![]() ��2Fe+H2����
��2Fe+H2����

����Ŀ��I������������Ӧ��
��Ӧ��![]()
![]() =a kJ/molƽ�ⳣ��ΪK1
=a kJ/molƽ�ⳣ��ΪK1
��Ӧ��![]()
![]() ƽ�ⳣ��ΪK2
ƽ�ⳣ��ΪK2
��Ӧ��![]()
![]() =b kJ/molƽ�ⳣ��ΪK3
=b kJ/molƽ�ⳣ��ΪK3
�ڲ�ͬ�¶��£�����K1��K2��ֵ���£�
T/�� | 700 | 800 |
K1 | 2.38 | 2.56 |
K2 | 0.80 |
(1) ![]()
![]() ==____________________
==____________________
(2)K1�ı���ʽΪ____________�����ݷ�Ӧ�����������Ƶ���K1��K2��K3�Ĺ�ϵʽK3=______________��
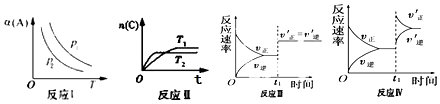
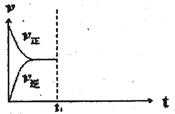
(3)�ں��º�ѹ�ܱ�������ͨ��CO��H2O��1mol������Ӧ�ڣ�����Ӧ�ﵽƽ���ά���¶���ѹǿ���䣬t1ʱ��ͨ���1mol��CO��H2O�Ļ�����壬������ͼ�л�������v�������棨v������Ӧ������t1����ʱ��t�仯������ͼ_______��
�����ݻ���ͬ�������ܱ�������(װ�е�����ij�ִ���)���ֱ����ͬ����NOx��C3H6���ڲ�ͬ�¶��£�ͬʱ�������·�Ӧ��
18NO(g)+2C3H6(g) 9N2(g)+6CO2(g)+6H2O(g)��
18NO2(g)+4C3H6(g) 9N2(g)+12CO2(g)+12H2O(g)��
���ֱ���t��ʱ�ⶨ����NOxת���ʣ����ͼ������ͼ��ʾ��
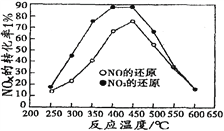
�ٴ�ͼ�п��Եó��Ľ�����
����һ���ӲⶨNOxת���������жϣ���ͬ�¶���NOת��Ч�ʱ�NO2�ĵ͡�
���۶���________________________________________________________
��������NO2��C3H6��Ӧ�У����NO2ת���ʵĴ�ʩ��_____________��(����)
A��������� B�������¶� C.�����H
���³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.6��c(H2CO3)=1.5��10 -5 mol/L��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3HCO3��+ H+ ��ƽ�ⳣ��K1=___________��(��֪��10-5.60=2.5��10-6 )