��Ŀ����
�������������̼���ŵ�һ�ַ����ǣ���ȼú�糧����Ȼ���糧�ŷ����л���CO2������CH4�Ƚ�¯������H2��Ӧ����CH3OH��
CO2��g��+3H2��g��
CH3OH��g��+H2O��g��
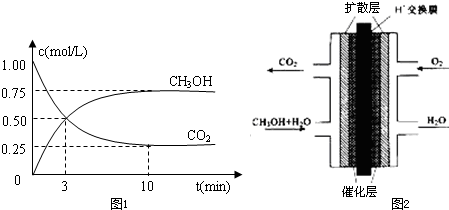
��1��500��ʱ�������Ϊ1L���ݻ��̶����ܱ������У�����1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ1��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��=______mol?L-1?min-1��
�ڸ÷�Ӧ��ƽ�ⳣ��K=______������һλС������ƽ��ʱH2��ת����Ϊ______��
����֪�¶����ߣ�Kֵ��С�����д�ʩ����ʹ
�������______��
A�������¶�B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з���D���ٳ���1molCO2��3molH2
��2��CH3OH��Ϊ��ȼ��ȼ�ϻ�ͨ��ȼ�ϵ�������������ɼ���CO2�ŷŶ��45%��
����֪��
CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H1=+49.0kJ?mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
��ӦCH3OH��g��+
O2��g���TCO2��g��+2H2��g���ġ�H=______kJ?mol-1��
�ڼ״����ӽ���Ĥȼ�ϵ�صĽṹʾ��ͼ��ͼ2����֪H+����ͨ��O2�ĵ缫���״�����______��������������������������ĵ缫��ӦΪ______��
CO2��g��+3H2��g��
| һ������ |
��1��500��ʱ�������Ϊ1L���ݻ��̶����ܱ������У�����1molCO2��3molH2�����CO2��CH3OH��g����Ũ����ʱ��仯��ͼ1��ʾ��

�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v��H2��=______mol?L-1?min-1��
�ڸ÷�Ӧ��ƽ�ⳣ��K=______������һλС������ƽ��ʱH2��ת����Ϊ______��
����֪�¶����ߣ�Kֵ��С�����д�ʩ����ʹ
| n(CH3OH) |
| n(CO2) |
A�������¶�B������He��g����ʹ��ϵѹǿ����
C����H2O��g������ϵ�з���D���ٳ���1molCO2��3molH2
��2��CH3OH��Ϊ��ȼ��ȼ�ϻ�ͨ��ȼ�ϵ�������������ɼ���CO2�ŷŶ��45%��
����֪��
CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H1=+49.0kJ?mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
��ӦCH3OH��g��+
| 1 |
| 2 |
�ڼ״����ӽ���Ĥȼ�ϵ�صĽṹʾ��ͼ��ͼ2����֪H+����ͨ��O2�ĵ缫���״�����______��������������������������ĵ缫��ӦΪ______��
��1������ͼ��֪��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L����v��CH3OH��=
=0.075mol/��L��min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��H2��=3v��CH3OH��=0.075mol/��L��min����3=0.225mol/��L��mon����
�ʴ�Ϊ��0.225��
��CO2��g��+3H2��g��
CH3OH��g��+H2O��g��
��ʼ����mol/L�� 1 3 0 0
�仯����mol/L�� 0.75 2.25 0.75 0.75
ƽ������mol/L�� 0.25 0.75 0.75 0.75
K=
=5.3
ƽ��ʱH2��ת����=
��100%=75%��
�ʴ�Ϊ��5.3��75%��
���¶����ߣ�Kֵ��С��˵����Ӧ �Ƿ��ȷ�Ӧ��ʹn��CH3OH��/n��CO2������Ӧ��ȡ��ʩ��ʹƽ��������Ӧ�ƶ���������ֻ���������̼�ͼ״�������
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�ƶ���n��CH3OH��/n��CO2����С����A����
B������He��g����ʹ��ϵѹǿ�����������ݻ����䣬��Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���n��CH3OH��/n��CO2�����䣬��B����
C����H2O��g������ϵ�з��룬ƽ��������Ӧ�ƶ���n��CH3OH��/n��CO2������C��ȷ��
D���ٳ���1molCO2��3molH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���n��CH3OH��/n��CO2������D��ȷ��
�ʴ�Ϊ��CD��
��2����a��CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H1=+49.0kJ?mol-1
b��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
���ݸ�˹���ɼ��㣬a+
b�õ�����ӦCH3OH��g��+
O2��g���TCO2��g��+2H2��g����H=-192.8KJ/mol��
�ʴ�Ϊ��-192.8��
�ڼ״�ȼ�ϵ���У��״���CԪ�صĻ��ϼ����ߣ���״�Ϊ�����������Ϊ�ᣬ�״�ʧȥ�������ɶ�����̼���缫��ӦΪCH3OH+H2O-6e-=CO2+6H+�����������õ���������ˮ���缫��ӦΪ��O2+4H++4e-�T2H2O��
�ʴ�Ϊ������O2+4H++4e-�T2H2O��
| 0.75mol/L |
| 10min |
�ʴ�Ϊ��0.225��
��CO2��g��+3H2��g��
| һ������ |
��ʼ����mol/L�� 1 3 0 0
�仯����mol/L�� 0.75 2.25 0.75 0.75
ƽ������mol/L�� 0.25 0.75 0.75 0.75
K=
| 0.75��0.75 |
| 0.25��0.753 |
ƽ��ʱH2��ת����=
| 2.25mol/L |
| 3mol/L |
�ʴ�Ϊ��5.3��75%��
���¶����ߣ�Kֵ��С��˵����Ӧ �Ƿ��ȷ�Ӧ��ʹn��CH3OH��/n��CO2������Ӧ��ȡ��ʩ��ʹƽ��������Ӧ�ƶ���������ֻ���������̼�ͼ״�������
A���÷�Ӧ����Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�ƶ���n��CH3OH��/n��CO2����С����A����
B������He��g����ʹ��ϵѹǿ�����������ݻ����䣬��Ӧ������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���n��CH3OH��/n��CO2�����䣬��B����
C����H2O��g������ϵ�з��룬ƽ��������Ӧ�ƶ���n��CH3OH��/n��CO2������C��ȷ��
D���ٳ���1molCO2��3molH2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���n��CH3OH��/n��CO2������D��ȷ��
�ʴ�Ϊ��CD��
��2����a��CH3OH��g��+H2O��g���TCO2��g��+3H2��g����H1=+49.0kJ?mol-1
b��2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
���ݸ�˹���ɼ��㣬a+
| 1 |
| 2 |
| 1 |
| 2 |
�ʴ�Ϊ��-192.8��
�ڼ״�ȼ�ϵ���У��״���CԪ�صĻ��ϼ����ߣ���״�Ϊ�����������Ϊ�ᣬ�״�ʧȥ�������ɶ�����̼���缫��ӦΪCH3OH+H2O-6e-=CO2+6H+�����������õ���������ˮ���缫��ӦΪ��O2+4H++4e-�T2H2O��
�ʴ�Ϊ������O2+4H++4e-�T2H2O��

��ϰ��ϵ�д�
�����Ŀ