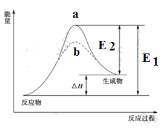
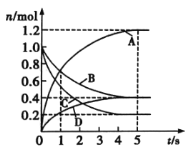
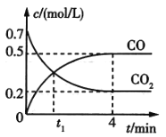
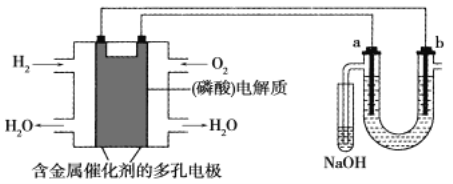
��Ŀ����
����Ŀ�����������֣�NASA������ŵ��ʿ�ҵ���һ�ֱȶ�����̼��Ч104���ġ������������塱ȫ�����飨C3F8������������䡰���һ����ǡ�ʹ���Ϊ�ڶ�������ļƻ����й�ȫ�������˵����ȷ���ǣ� ��
A.ȫ�����飨C3F8�������м��м��Լ����зǼ��Լ�
B.ȫ�����飨C3F8���ĵ���ʽΪ��
C.��ͬѹǿ�£��е㣺C3F8��C3H8
D.����������̼ԭ�ӿ��ܴ���ͬһֱ����
���𰸡�A
��������
A��ȫ�����飨C3F8���Ľṹ��ʽΪCF3CF2CF3��C��C֮��Ϊ�Ǽ��Թ��ۼ���C��F֮��Ϊ���Թ��ۼ���A��ȷ��
B��ȫ��������Fԭ��Ӧ����8�����ȶ��ṹ��B����
C��C3F8��C3H8�ṹ���ƣ�C3F8����Է���������C3H8����Է���������C3F8�з��Ӽ���������C3H8ǿ���۷е�C3F8��C3H8�ߣ�C����
D��C3F8ΪC3H8��ȫ��ȡ�������Cԭ��Ӧ�ʾ��״����������ͬһֱ���ϣ�D����
��ѡA��

��ϰ��ϵ�д�
�����Ŀ