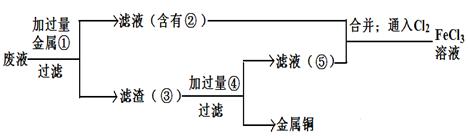
��Ŀ����
ijѧУ��ѧ��ȤС��Ϊ̽��������������ۺ����ã�ר�����ʵ���ú�����������ͭ�ĺϽ���ȡ������AlCl3��Һ�͵������壨CuSO4?5H2O������ʵ�鷽�����£�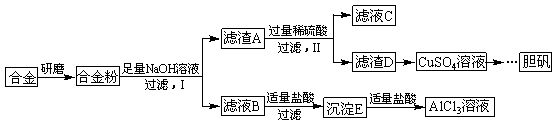
��ش��������⣺
��1�����Ͻ���ĥ�ɺϽ�۵�Ŀ���� ��
��2���ֱ�д��������з�����Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
��3������ҺBת��Ϊ����E�Ĺ�������������������ƣ��ɽ����������������Ϊͨ��һ�����壬������ĵ���ʽΪ ��д�������Լ�����������������ɳ���E��������Ӧ�����ӷ���ʽ�� ��
��4����С���Ա�������л�֪H2O2��һ����ɫ��������������D�м���ϡ�����H2O2���Ƶ�CuSO4���÷�Ӧ���ܻ�ѧ����ʽΪ ��
��1������Ӧ��ĽӴ������ʹ��Ӧ���ʼӿ죨��ʹ��Ӧ����֣�
��2����2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2�� ��Fe+H2SO4=FeSO4+H2��
��3��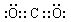 [Al(OH)4]-+CO2=Al(OH)3��+HCO3-
[Al(OH)4]-+CO2=Al(OH)3��+HCO3-
��4��Cu+H2O2+H2SO4=CuSO4+2H2O
�������������������ͼ��֪�Ͻ��м���NaOH��Һ������ӦΪ��2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2������ҺB�к���Na[Al(OH)4]��NaOH������A�к�������ͭ����ҺB�м��������������ɵij���EΪAl(OH)3
��1������Ӧ��ĽӴ������ʹ��Ӧ���ʼӿ죨��ʹ��Ӧ����֣�
��2����2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2�� ��Fe+H2SO4=FeSO4+H2�� ��3����[Al(OH)4]-����ü������ᣬ��Al(OH)3���������ᣬ��ͨ������ΪCO2���ʴ�Ϊ��
��3����[Al(OH)4]-����ü������ᣬ��Al(OH)3���������ᣬ��ͨ������ΪCO2���ʴ�Ϊ��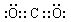 [Al(OH)4]-+CO2 Al(OH)3��+HCO3-
[Al(OH)4]-+CO2 Al(OH)3��+HCO3- ��4��Cu+H2O2+H2SO4 CuSO4+2H2O
��4��Cu+H2O2+H2SO4 CuSO4+2H2O
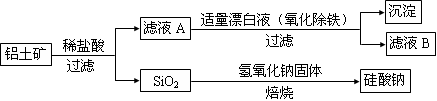
���㣺�������ʵ����ʼ�ת�������֪ʶ��

�ú���A12O3��SiO2������FeO·xFe2O3�������Ʊ�A12(SO4)3·18H2O����������������(���ֲ�����������):
��.�������м������ϡH2SO4������;
��.����Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
��.���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ;
��.����MnSO4���Ϻ�ɫ��ʧ�����ˣ�
��.Ũ�����ᾧ�����룬�õ���Ʒ��
H2SO4�ܽ�A12O3�����ӷ���ʽ��
��KMnO4 ����Fe2+�����ӷ���ʽ���������� MnO4-+��Fe2++�� =
MnO4-+��Fe2++�� = Mn2++��Fe3+ +��
Mn2++��Fe3+ +��
��ʽ���������� ,���������� ��
��3����֪�������������������pH ks5u
| | Al��OH��3 | Fe��OH��2 | Fe��OH��3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�ģ� ��
��֪:һ�������£�MnO4 - ����Mn2+��Ӧ����MnO2��
�� �� �� �ij����м���ŨHCI�����ȣ���˵�������д���MnO2�������� ��
�ڢ� �м���MnSO4��Ŀ���� ��
 ��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺
��ϡ��Һ�У���ʹ���ַ�Ӧ��������Ļ�ԭ����ֻ��NO���Իش��������⣺ ��Cu
��Cu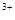 ��Fe
��Fe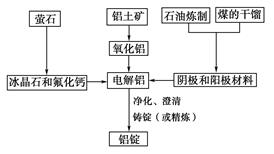
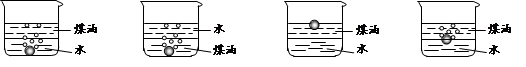
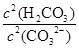 ����������С�����䡱��
����������С�����䡱��