��Ŀ����
����Ŀ������N��P��Fe��Ti��Ԫ�ص����Ͳ������Ź㷺����;��
(1)��̬Feԭ��δ�ɶԵ�����Ϊ______������̬Tiԭ�ӵļ۵����Ų�ͼ��_____________��
(2)�����������ѧ�ģ�FuNvio Cacace���˻���˼��������о������N4���ӣ����е�ԭ�ӵĹ���ӻ���ʽΪ__________________________��
(3)�Ƚ���̬�⻯���(PH3)�Ͱ�(NH3)�ļ��ǣ�PH3________NH3(����ڡ�����С�ڡ����ڡ�)����Ҫԭ��Ϊ____________________________________________________________��
(4)����Ľṹ����M�ܴ���ϩ����ϩ������ϩ�ľۺϣ���ṹ��ͼ��ʾ��

�����M��Ԫ���У��縺��������___________(������)��
��M���_________(����)��
A ���� B ����
C ���Ӽ� D ��λ��

(5)��֪���ʯ�ľ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ����ͼB��ʾ�������������������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼӦ����ͼ__________(����)��

(6)ij�ִ��Ե������ľ����ṹ��ͼ��ʾ�����о�����ԭ���������ԭ�ӵĸ���Ϊ____________�������������ױ߳�Ϊa cm����Ϊc cm�������ִ��Ե������ľ����ܶ�Ϊ__________g��cm-3(�ú�a��c��NA�ļ���ʽ��ʾ)��
���𰸡�4 ![]() sp3 С�� �縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�� �� ABD A 12
sp3 С�� �縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ�� �� ABD A 12 ![]()
��������
(1) FeΪ26��Ԫ�أ���̬Fe�ĺ�������Ų�ʽΪ1s22s22p63s23p63d64s2����̬ԭ��δ�ɶԵ�����Ϊ4����TiΪ22��Ԫ�أ���̬Ti�ĺ�������Ų�ʽΪ1s22s22p63s23p63d24s2�����Ի�̬Tiԭ�ӵļ۵����Ų�ͼ��![]() ���𰸣�4��
���𰸣�4��![]() ��
��
��2��N4���ӵĿռ乹����P4���ƣ�4��Nԭ���γ��������幹�ͣ�ÿ��Nԭ���γ�3��N-N����������1�Թµ��Ӷԣ��ӻ������ĿΪ4����Nԭ�Ӳ�ȡsp3�ӻ����𰸣�sp3��
(3) ��Ϊ�縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ������PH3С��NH3���𰸣�С�ڣ��縺��Nǿ��P������ԭ�ӵĵ縺��Խ�ɼ����Ӷ�������ԭ��Խ�����ɼ����Ӷ�֮�����ԽС���ɼ����Ӷ�֮����ų��������DZ��
(4) �����M��Ԫ����Ti��C��H��O��Cl ������O�ķǽ�������ǿ���ǽ�����Խǿ�縺��Խ�����Ե縺�������������𰸣�����
��M����̼̼˫����̼̼������C��H����C��O���ȣ�����Ϊ![]() ���� ˫���к�1��
���� ˫���к�1��![]() ����1������������M�Ľṹ֪Ti��O֮������λ����û�����Ӽ�����ѡABD��
����1������������M�Ľṹ֪Ti��O֮������λ����û�����Ӽ�����ѡABD��
(5) )�ɽ��ʯ�ľ����ṹ��֪�����ʯ�ľ����൱��һ��������������ѻ�����һ��С�����������ѻ����������ľ���Ϊ���������ѻ������ݽ��ʯ�ľ���������Խ��ߴ�ֱ��ֽƽ���ϵ�ͶӰͼ֪���������ľ���������Խ��ߴ�ֱ��ֽ���ϵ�ͶӰͼΪA���𰸣�A��
(6) ���ݾ�̯�����ڵ����������У�����Nԭ����Ϊ2��Feԭ����Ϊ2��1/2+12��1/6+3=6�����Ե������Ļ�ѧʽFe3N��������ԭ���������ԭ�ӵĸ���Ϊ12���������ױ߳�Ϊacm����Ϊccm�����������![]() a2ccm3���������ִ��Ե������ľ����ܶ�Ϊ
a2ccm3���������ִ��Ե������ľ����ܶ�Ϊ![]() g��
g��![]() a2ccm3=
a2ccm3=![]() g/cm3���𰸣�12��
g/cm3���𰸣�12��![]() ��
��

 �������ͬ����ϰϵ�д�
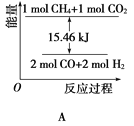
�������ͬ����ϰϵ�д�����Ŀ����֪��CO(g) + H2O(g) ![]() H2(g) + CO2(g) ��ƽ�ⳣ��K���¶ȵı仯���±�������˵����ȷ������ ��
H2(g) + CO2(g) ��ƽ�ⳣ��K���¶ȵı仯���±�������˵����ȷ������ ��
�¶�/�� | 400 | 500 | 830 | 1000 |
ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
A. �÷�Ӧ������Ӧ�����ȷ�Ӧ
B. ����ʱ����ѹǿ������Ӧ��������
C. 830��ʱ����Ӧ�ﵽƽ�⣬һ����c(CO)��c(CO2)
D. 400��ʱ������CO2���ʵ���Խ�࣬ƽ�ⳣ��KԽ��