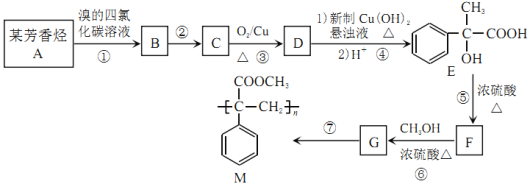
��Ŀ����
����Ŀ��ij����С���ͬѧ��ʵ����������װ����ȡ��������������Ҫ�������£�
����30mL�Ĵ��Թ�A�а������2��3��2�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�ڰ���ͼ���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5~10min��
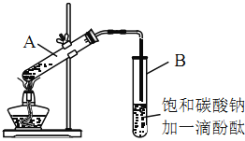
�۴��Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㡣
�ܷ�������������㡢ϴ�ӡ����
��֪�������ݣ�
���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g/cm3�� |
�Ҵ� | ��117.0 | 78.0 | 0.79 |
���� | 16.6 | 117.9 | 1.05 |
�������� | ��83.6 | 77.5 | 0.90 |
Ũ���ᣨ98%�� | �D�D | 338.0 | 1.84 |
��ش��������⣺
��1�����Ƹû����Һʱ���������������ʵ��Ⱥ�˳����___________��д����ȡ���������Ļ�ѧ����ʽ��___________��
��2����ʵ���У�Ũ�����������___________��
��3������ʵ���б���̼������Һ��������___________������ĸ����
A ���ղ����Ҵ�
B �����
C ���������������ܽ�ȣ������ڷֲ�����
D �����������ɣ���������
��4�����������ҪС����ȼ��Ȳ���������Ҫ������___________��
��5���������B�Թ��ڵ��ϲ�������___________�����������ƣ���
��6��������з�������õ�����Ҫ������___________����ѡ�õĸ����Ϊ___________������ĸ����
A ��ʯ�� B NaOH����
C ��ʯ�� D ��ˮNa2SO4
���𰸡��Ҵ���Ũ���ᡢ���� CH3COOH+CH3CH2OH![]() CH3COOC2H5+H2O ��������ˮ�� ABC ��Ϊ��Ӧ���Ҵ�������ķе�ϵͣ����ô����ȣ���Ӧ������������������ʧԭ�ϣ��¶ȹ������ܷ�����������Ӧ �������� ��Һ©�����ձ� D
CH3COOC2H5+H2O ��������ˮ�� ABC ��Ϊ��Ӧ���Ҵ�������ķе�ϵͣ����ô����ȣ���Ӧ������������������ʧԭ�ϣ��¶ȹ������ܷ�����������Ӧ �������� ��Һ©�����ձ� D
��������
��1��Ϊ��ֹ��Һ�ɽ���Ӧ���ܶȴ��Һ����뵽�ܶ�С��Һ���У������ӷ�����ȴ���ټ������ᣬ�������ʵļ���˳���Ҵ���Ũ���ᡢ���������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ������ʽΪ��CH3COOH+CH3CH2OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��2����ʵ���У��Ҵ������ᷢ��������Ӧʱ��Ũ�������������ã��ӿ�������Ӧ�����ʣ�����Ϊ�÷�Ӧ�ǿ��淴Ӧ��Ũ������ˮ���Դٽ�ƽ��������Ӧ������У�����Ũ���ỹ����ˮ�������á�
��3�������Ҵ��������ӷ������������������к����Ҵ�������Ҵ�������ˮ���ܱ�����̼������Һ���գ�����������ԣ����뱥��̼������Һ��Ӧ���������ƣ��������������ڱ���̼������Һ�е��ܽ�ȣ������ڷֲ���������Ϊ��ABC����
��4�����������ҪС����ȼ��Ȳ���������Ҫ�����ǣ���Ϊ��Ӧ���Ҵ�������ķе�ϵͣ����ô����ȣ���Ӧ������������������ʧԭ�ϣ��¶ȹ������ܷ�����������Ӧ��
��5���������������ڱ���̼������Һ���ܶȱ�ˮС������ζ���������B�Թ��ڵ��ϲ�����������������
��6��������з�������Ƿ�Һ���õ�����Ҫ�����Ƿ�Һ©�����ձ���
������������������ˮ�����Ƴ�ȥ������ˮ����ˮ��������ˮ�γ������ƽᾧˮ�������ѡ����ʯ�ҡ���ʯ�ҡ�NaOH���Է����������ڼ���������ˮ�⣬��ѡD��

����Ŀ������ͼ����ʾװ�ý���ʵ�飬���ܵó���Ӧ������ǣ� ��
ѡ�� | ��Һ�� | ����� | ��Һ�� | ʵ������ |
|
A | Ũ��ˮ | ��ʯ�� | ��̪��Һ | ��̪��Һ��� | |
B | Ũ���� |
|
| ��������ɫ���� | |
C | Ũ���� |
|
| �Թ��Ϸ���������ɫ���� | |
D | ϡ���� |
|
| ������ɫ���� |
A.AB.BC.CD.D