��Ŀ����
����Ŀ��������Դ�ḻ����ˮˮ��Դ�����úͺ�ˮ��ѧ��Դ����ҪΪNaCl��MgSO4��K��Br��Ԫ�أ������þ��зdz�������ǰ����
��1�����ú�ˮ������ȡ���þ����ȡ�������£�

����ȡ��Ĺ����У�����2��Br- �� Br2ת����Ŀ����_____���������з�����Ӧ�����ӷ���ʽ��________��
�ڴ�MgCl2��Һ�еõ�MgCl2.6H2O�������Ҫ������__________��_________�����ˡ�ϴ�ӡ����
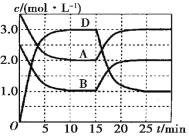
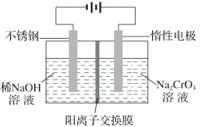
��2��![]()
�����չ������õ���ʵ�������������Ǽܡ��ƾ��ơ�����ǯ��_____��______��
�ڲ����������õ�����������������������_______________��
�����ữ���ˮ��Һ��������3% H2O2��Һ��������Ӧ�Ļ�ѧ����ʽΪ________��
�ܲ������Ƿ�Һ�����������___________����������___________
���𰸡�����Ԫ�ؽ��и��� SO2 + Br2 + 2H2O === 4H+ + SO42- + 2Br- ����Ũ�� ��ȴ�ᾧ ���� ������ ���衢���� 2I-+2H++H2O2==I2+2H2O ��ȡ ����
��������
(1)���������ú�ˮ������ȡ���þ����������ȡ��Ĺ����У�����2��Br-��Br2ת����Ŀ���Ǹ���ĵõ��嵥�ʣ���ȡ���̶���Ԫ�ؽ��и�������������ͨ����Ƕ������������Ǻ��嵥�ʷ�Ӧ���������ӣ����������б����������õ�������嵥�ʣ��������з�Ӧ�����ӷ���ʽΪ��SO2+Br2+2H2O=4H++2Br-+SO42-���ʴ�Ϊ������Ԫ�ؽ��и�����SO2+Br2+2H2O=4H++2Br-+SO42-��
�ڴ�MgCl2��Һ�еõ�MgCl26H2O�������Ҫ����������Ũ������ȴ�ᾧ������ϴ�ӵõ����ʴ�Ϊ������Ũ������ȴ�ᾧ��
(2)�����չ������õ���ʵ�������������Ǽܡ��ƾ��ơ�����ǯ�������������ǣ��ʴ�Ϊ�������������ǣ�
�ڲ�����Ϊ�ܽ⡢���ˣ����õ���������������������Ϊ���衢�������ʴ�Ϊ�����衢������
�����ữ���ˮ��Һ��������3% H2O2��Һ�����������������ɵⵥ�ʣ���Ӧ�Ļ�ѧ����ʽΪ2I-+2H++H2O2==I2+2H2O���ʴ�Ϊ��2I-+2H++H2O2==I2+2H2O��
��������õ����ˮ��Һ��Ҫ�õ��ⵥ�ʣ�����ͨ����ȡ�ͷ�Һ���������Ƿ�Һ�������������ȡ����Һ��õ�����л���Һ������ͨ������ķ����������л��ܼ�����˲����������ʴ�Ϊ����ȡ������