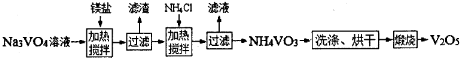
��Ŀ����
���Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ�ά����������ҵ�ϻ��շϷ�����������V2O5��VOSO4��K2SO4��SiO2���з�����Ҫ�������£�
��֪����1��V2O5��NH4VO3��Ϊ�����VOSO4��(VO2)2SO4��Ϊ�����
��2�� 2VO2++H2C2O4+2H+ = 2VO2+ + 2CO2��+ 2H2O
�ش��������⣺
��1��������ǰ�������Ŀ����_________________________��
��2���������з�����Ӧ�����ӷ���ʽΪ__________________________��
��3���������ı仯���̿ɼ�Ϊ(HA��ʾ�л���ȡ��)��
VOSO4 (ˮ��)+ 2HA���л��㣩 VOA2(�л��㣩+ H2SO4(ˮ��)���������п�ѡ������������ȡ��ԭ����_____________��
VOA2(�л��㣩+ H2SO4(ˮ��)���������п�ѡ������������ȡ��ԭ����_____________��
��4���������ữ��H2C2O4��Һ�ζ�(VO2)2SO4��Һ���Բⶨ����������Һ�к������IJ���Ϊ��ȡ10.0mL0.1mol/LH2C2O4��Һ����ƿ�У�����ָ�����������Һʢ���ڵζ����У��ζ����յ�ʱ�����Ĵ���Һ�����Ϊ10.0mL���ɴ˿�֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ_________g/L��
��5��V2O5���ý���(��Ca��Al)�Ȼ�ԭ����÷�����������Ȼ�ԭ�Ƶ÷��Ļ�ѧ����ʽΪ_______________��
��1�������Һ�Ӵ�������ӿ�������ʣ���߽�������2�֣�
��2��V2O5+ SO32��+4H+=2VO2+ + SO42��+2H2O��2�֣�
��3���������ᣬ��ʹƽ��������У�ʹVOSO4����ˮ�㣻��2�֣�
��4��10.2g/L ��2�֣�
��5��3V2O5+10Al 6V+5Al2O3��2�֣�
6V+5Al2O3��2�֣�
��������
�����������1��������巴Ӧ����������Һ�Ӵ�������ӿ�������ʣ���߽�������
��2������������л�ԭ�ԣ����������£��ܱ���������������������������ӣ����ӷ�Ӧ����ʽΪ��V2O5+ SO32��+4H+=2VO2+ + SO42��+2H2O
��3������H2SO4��H2SO4Ũ��������ۻ�ѧƽ���������ƶ���ʹVOSO4����ˮ����
��4��������Ŀ������Ϣ��2VO2+? ~ H2C2O4����֪(VO2)2SO4��Һ��Ԫ�صĺ���Ϊ��0.1mol/L��0.01L��2��51g/mol��0.01L= 10.2g/L��
��5������������������Ӧ���ɷ�������������ѧ����ʽΪ��3V2O5+10Al 6V+5Al2O3
6V+5Al2O3
���㣺���⿼�黯ѧ�������̵ķ���������ʽ����д����ѧ���㡣

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д���֪��V���γ�VO2+��VO2+��VO3-�ȶ������ӣ����ֺ���������ˮ�е��ܽ������±���ʾ��
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
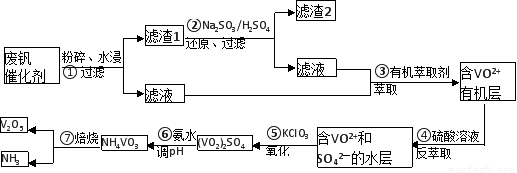
��ش���������
��1����ҵ�ϳ������ȷ�Ӧ����V2O5ұ������������д����Ӧ�Ļ�ѧ����ʽ
��2����Ӧ�ٵ����ӷ���ʽ��
��3���ڷ�Ӧ���У�ÿ����1mol��VO2��2SO4ת�Ƶ��ӵ���ĿΪ
��4�������ӽ��������У��������淴ӦVO2++2OH-?VO3-+H2O��
�÷�Ӧ�Ļ�ѧƽ�ⳣ��K �ı���ʽΪ
��5����Ӧ�۳�ַ�Ӧ�����NH4VO3��ʵ��������Ҫ��������Ϊ

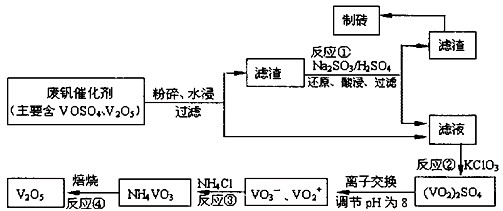
 ����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ
����a��c�����Ļ�ѧ����ʽ�ɱ�ʾΪ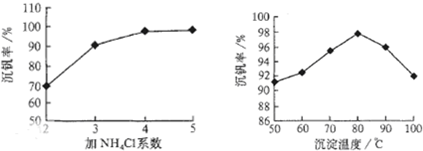
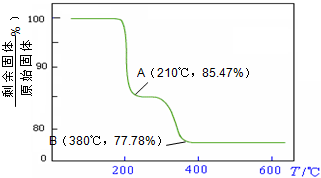 ��NH4VO3�ڷֽ������
��NH4VO3�ڷֽ������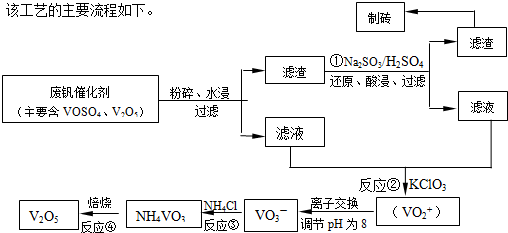
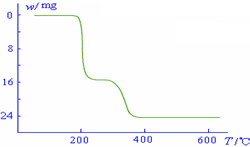 ������������ͼ��ʾ����NH4VO3�ڷֽ������
������������ͼ��ʾ����NH4VO3�ڷֽ������