��Ŀ����
��32.64gͭ��140mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2L����ش�
��1��NO�����Ϊ L��NO2�����Ϊ L��
��2��������������ȫ���ͷź�����Һ����VmL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ mol/L��
��3����ʹͭ�����ᷴӦ���ɵ�������NaOH��Һ��ȫ��ת��ΪNaNO3��������Ҫ30%��˫��ˮ
g��
��1��NO�����Ϊ L��NO2�����Ϊ L��
��2��������������ȫ���ͷź�����Һ����VmL a mol/L��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ mol/L��
��3����ʹͭ�����ᷴӦ���ɵ�������NaOH��Һ��ȫ��ת��ΪNaNO3��������Ҫ30%��˫��ˮ
g��
��1��5.8 5.4 ��2�� (av.10-3+0.5)/0.14 ��3��57.67
���������ͭ��һ��Ũ�ȵ����ᷴӦ���漰�Ļ�ѧ����ʽΪ��
Cu+4HNO3(Ũ)=Cu(NO3)2+2H2O+NO2
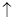 ��3Cu+8HNO3(ϡ)=3Cu(NO3)2+4H2O+2NO
��3Cu+8HNO3(ϡ)=3Cu(NO3)2+4H2O+2NO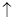
��1����NO�����Ϊ
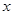 L����NO2�����Ϊ
L����NO2�����Ϊ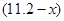 L������������ʽ��֪����ϡ���ᷴӦ��ͭ������Ϊ
L������������ʽ��֪����ϡ���ᷴӦ��ͭ������Ϊ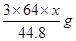 ������Ũ���ᷴӦ��ͭ������Ϊ
������Ũ���ᷴӦ��ͭ������Ϊ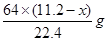 ����˿ɵ����µ�ʽ��
����˿ɵ����µ�ʽ��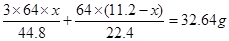 �����
�����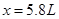 �����NO�����Ϊ5.8L��NO2�����Ϊ5.4L��
�����NO�����Ϊ5.8L��NO2�����Ϊ5.4L����2����Һ��ͭ���ӵ����ʵ���
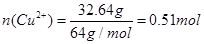 �����ԭ��������ʵ���Ũ��
�����ԭ��������ʵ���Ũ��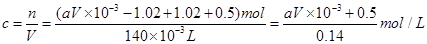
��3�������ķ�ӦΪ2NO2+H2O2=2HNO3��2NO+3H2O2=2HNO3+2H2O������NO2��Ӧ��H2O2������Ϊ
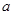 ����NO��Ӧ��H2O2������Ϊ
����NO��Ӧ��H2O2������Ϊ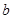 ����ɵ����¹�ϵʽ��
����ɵ����¹�ϵʽ��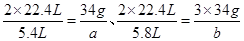 �����
�����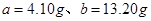 �������Ҫ30%��˫��ˮ������Ϊ
�������Ҫ30%��˫��ˮ������Ϊ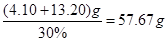 ��
�����������⿼����ͭ�����ᷴӦ�����漰���ļ��㣬���ڻ����⡣����Ĺؼ�������ȷд����ѧ����ʽ���ڼ���Ĺ�����Ӧע���ϵʽ�����غ㷨��Ӧ�á�

��ϰ��ϵ�д�
 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ