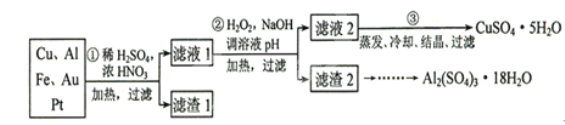
��Ŀ����
����Ŀ�������ֽ�������A��B��C������A����ɫ��ӦΪ��ɫ��B��C�dz������������ֽ�������A��B��C��������ס��ҡ���������D��E��F��G��H֮�䷢������ת����ϵ(ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û�б��)�������������Ϣ�ش��������⣺
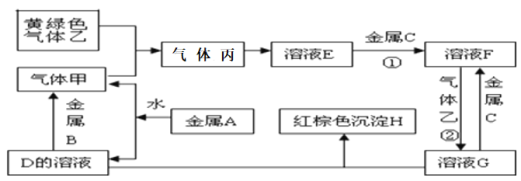
(1)д���������ʵĻ�ѧʽ����_________��H__________��
(2)д����Ӧ�ڵ����ӷ���ʽ______________________��
(3)д������A��ˮ��Ӧ�����ӷ���ʽ___________________________��
(4)д������B��D����Һ��Ӧ�����ӷ���ʽ____________________��
(5)F��D��Һ��Ӧ��IJ����ڿ�����ת��ΪH�Ļ�ѧ����ʽ___________������Ϊ_________________��
���𰸡�Cl2 Fe(OH)3 2Fe2++Cl2=2Fe3++2Cl- 2Na+2H2O=2Na++2OH-+H2�� 2Al+2OH-+2H2O=2AlO2-+3H2�� 4Fe(OH)2+O2+2H2O=4Fe(OH)3 ��ɫ������Ϊ����ɫ������Ϊ���ɫ
��������
����ɫ����Ϊ��������������ܷ�Ӧ��˵�������ΪH2���������ΪHCl������A����ɫ��Ӧ�Ի�ɫ��˵��A��Na����DΪNaOH������B��NaOH��Һ��Ӧ������������BΪAl�����ɫ����HΪFe(OH)3����G�к���Fe3������ҺF��������Ӧ����Fe3������F�к���Fe2����C�dz����������ܺ����ᷴӦ������ҺF�������CΪFe���ݴ˽��
�������Ϸ�����֪A��Na��BΪAl��CΪFe��DΪNaOH����ҺE�����ᣬ��ҺF���Ȼ���������ҺG���Ȼ�����H�����������������ΪH2������ɫ������Ϊ�����������ΪHCl����
��1�������Һ�H�Ļ�ѧʽ�ֱ���Cl2��Fe(OH)3��
��2���������Ϸ�����֪��Ӧ�ڵ����ӷ���ʽΪ2Fe2++Cl2=2Fe3++2Cl-��
��3������A���ƣ���ˮ��Ӧ�����ӷ���ʽΪ2Na+2H2O=2Na++2OH-+H2����
��4��������������������Һ��Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��5��F��D��Һ��Ӧ��IJ������������������ױ�����Ϊ�������������ڿ�����ת��ΪH�Ļ�ѧ����ʽΪ4Fe(OH)2+O2+2H2O=4Fe(OH)3������Ϊ��ɫ������Ϊ����ɫ������Ϊ���ɫ��