��Ŀ����
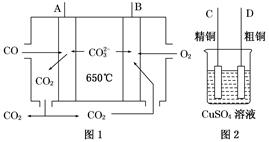
��1����ͼ1��һ������ȼ�ϵ�أ�����COΪȼ�ϣ�һ��������Li2CO3��Na2CO3���ڻ����Ϊ����ʣ�ͼ2�Ǵ�ͭ������װ��ͼ������ȼ�ϵ��Ϊ��Դ���д�ͭ�ľ���ʵ�顣
�ش��������⣺
��д��A�������ĵ缫��Ӧʽ______________________________________��
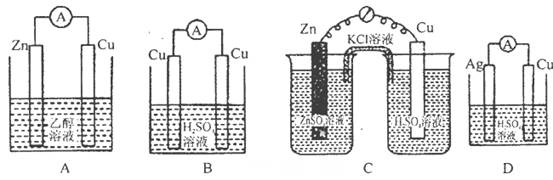
��Ҫ��ȼ�ϵ��Ϊ��Դ���д�ͭ�ľ���ʵ�飬��B��Ӧ��________������(�C����D��)��
�۵����ı�״����2.24 L COʱ��C�缫�������仯Ϊ________��
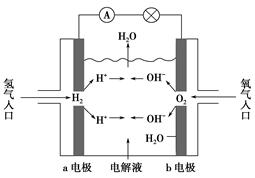
��2����ҵ�ϣ������������������KOH��Һ�Ʊ�K2FeO4��
�ٵ������У�OH����________(�����������)���ƶ��������ĵ缫��ӦʽΪ____________________________��
����������28 g Fe�ܽ⣬�����������������ڱ�״���µ����Ϊ________L��

�ش��������⣺
��д��A�������ĵ缫��Ӧʽ______________________________________��
��Ҫ��ȼ�ϵ��Ϊ��Դ���д�ͭ�ľ���ʵ�飬��B��Ӧ��________������(�C����D��)��
�۵����ı�״����2.24 L COʱ��C�缫�������仯Ϊ________��
��2����ҵ�ϣ������������������KOH��Һ�Ʊ�K2FeO4��
�ٵ������У�OH����________(�����������)���ƶ��������ĵ缫��ӦʽΪ____________________________��
����������28 g Fe�ܽ⣬�����������������ڱ�״���µ����Ϊ________L��
��1����CO��2e����CO32-=2CO2����D��������6.4 g
��2��������Fe��6e����8OH��=FeO42-��4H2O
��33.6
��2��������Fe��6e����8OH��=FeO42-��4H2O
��33.6
��1����ͼ��֪AΪ������BΪ������DΪ������CΪ������
��2�����ݵ��ԭ�����������������ƶ�����������������Ӧ����ȷ��������ӦʽΪFe��6e����8OH��=FeO42-��4H2O��������ӦʽΪ2H����2e��=H2����
��2�����ݵ��ԭ�����������������ƶ�����������������Ӧ����ȷ��������ӦʽΪFe��6e����8OH��=FeO42-��4H2O��������ӦʽΪ2H����2e��=H2����

��ϰ��ϵ�д�
�����Ŀ