��Ŀ����
�����йص������Һ��ϵ��ȷ����
A���ֱ���pH=2������ʹ�����Һ�У���ˮϡ�͵�pH=4������ˮ��������
B�������ʵ���Ũ�ȵ��Ȼ��ƺʹ�������Һ�У�����ϵ��c(Na��)= c(Cl��)+c(CH3COO��)
C����NaHSO3��Һ���ɴ�С��˳��Ϊ��c(Na��)��c(HSO3��)��c(SO32��)��c(H2SO3)
D�������������H2A��H2B��������ʵ���Ũ�ȵ������Na2A��Na2B��Һ�У���������Ŀǰ�ߴ��ں���
A���ֱ���pH=2������ʹ�����Һ�У���ˮϡ�͵�pH=4������ˮ��������
B�������ʵ���Ũ�ȵ��Ȼ��ƺʹ�������Һ�У�����ϵ��c(Na��)= c(Cl��)+c(CH3COO��)
C����NaHSO3��Һ���ɴ�С��˳��Ϊ��c(Na��)��c(HSO3��)��c(SO32��)��c(H2SO3)
D�������������H2A��H2B��������ʵ���Ũ�ȵ������Na2A��Na2B��Һ�У���������Ŀǰ�ߴ��ں���
C
���������ѡ��A������Ϊǿ�ᣬ����Ϊ���ᣬ��ˮϡ��ʱ��������ˮ�������ѡ��B������������غ��c(Na��)+c(H��)= c(Cl��)+c(CH3COO��)��ѡ��D��H2A����ǿ��H2B��B2-�����������ӽ�ϵ�����ǿ��A2-���ӣ����Na2A�ļ�������Na2B���ٸ���c(Na+)+c(H+)=c(A2-)+c(OH-) ,
c(Na+)+c(H+)=c(A2-)+c(OH-),Na+Ũ����ͬ��A�е�c(H+)����B��c(H+),�ɵ�Na2A��Һ�е�����������
����������ؼ����ڻ����������غ㣬����Һ���������ж�����Ũ�ȵĴ�С��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
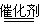 2CO2��N2
2CO2��N2 2CrO42��+2H+ƽ�����ƣ�����Ag2CrO4�������٣�Ӱ��ʵ����
2CrO42��+2H+ƽ�����ƣ�����Ag2CrO4�������٣�Ӱ��ʵ���� ʵ������
ʵ������