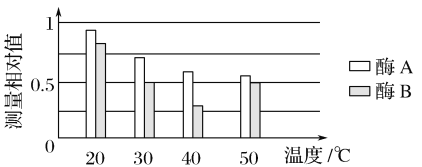
��Ŀ����
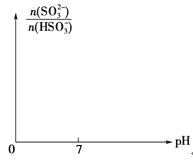
����Ŀ�������£�����0.1mol/L��NH4HCO3��Һ��pH��7.8����֪��������̼�������ķֲ�������ƽ��ʱij������Ũ��ռ������Ũ��֮�͵ķ�������pH�Ĺ�ϵ����ͼ��ʾ������˵����ȷ���ǣ�

A������Һ��pH��9ʱ����Һ�д������й�ϵ��c��NH4������c��HCO3������c��NH3��H2O����c��CO32����
B��NH4HCO3��Һ�д��������غ��ϵ��c��NH4������c��NH3��H2O����c��H+����c��OH������2c��CO32������c��H2CO3��
C��������Һ����εμ���������ʱNH4����HCO3��Ũ����С
D��ͨ��������֪������Kb��NH3��H2O����Ka1��H2CO3��
���𰸡�D
��������
���������A������ʾ��ͼ��֪������Һ��pH��9ʱ����Һ�д������й�ϵ��c��HCO3������c��NH4������c��NH3��H2O����c��CO32����������B�����������غ�ɵ�NH4HCO3��Һ�д��������غ��ϵ��c��NH4������c��NH3��H2O����c��CO32������c��H2CO3����c��HCO3-������ΪNH4+����ˮ�ⷴӦ������c��HCO3-����c��CO32������c��OH������c��H+������C���й�ϵʽ����D����Ϊ0.1mol/L��NH4HCO3��Һ��pH��7.8��˵��HCO3��ˮ��̶ȴ���NH4+��ˮ��̶ȣ�����Խ��Խˮ��Ĺ��ɿɵã�Kb��NH3��H2O����Ka1��H2CO3������ȷ��

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�