��Ŀ����
17����������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na+��NH4+��Mg2+��Al3+��SO42-��NO3-��Cl-��ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ������������Һ����Ʋ��������ͼ��ʵ�飺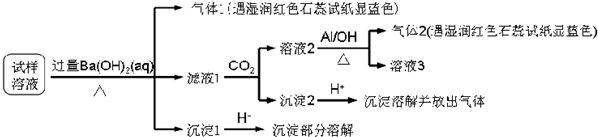
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ���ǣ�������
| A�� | �����п϶�����NH4+��Mg2+��SO42-��NO3- | |
| B�� | �����п��ܴ���Na+��Cl- | |
| C�� | ������һ������Al3+ | |
| D�� | �������п��ܴ���NaNO3��NH4Cl��MgSO4 |
���� ������Һ�м������Ba��OH��2�����ȣ����ɵ�����1��������1������NH3���������к���NH4+������Һ��ͨ��CO2���õ���Һ2������2����Һ2�м���Al��NO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������2����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-����Һ1��ͨ��CO2���õ�����2�������2�м����ᣬ�����ܽⲢ�ų����壬˵������2��̼�ᱵ��������̼���Σ�����1��������������ܽ⣬���ᱵ�������ᣬ˵��ԭ����Һ�к���SO42-���ܺ���Ba��OH��2��Ӧ������������ij����������ṩ�����ӿ�֪�ó���ΪMg��OH��2��������Һ�к���Mg2+��������ѡ��������
��� �⣺������Һ�м������Ba��OH��2�����ȣ����ɵ�����1��������1������NH3���������к���NH4+������Һ��ͨ��CO2���õ���Һ2������2����Һ2�м���Al��NO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������2����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-����Һ1��ͨ��CO2���õ�����2�������2�м����ᣬ�����ܽⲢ�ų����壬˵������2��̼�ᱵ��������̼���Σ�����1��������������ܽ⣬���ᱵ�������ᣬ˵��ԭ����Һ�к���SO42-���ܺ���Ba��OH��2��Ӧ������������ij�������������֪���ó���ΪMg��OH��2��������Һ�к���Mg2+��
A���������Ϸ�����֪��Һ�к���NH4+��Mg2+��SO42-��NO3-����A��ȷ��
B������ʵ����ȷ���Ƿ���Na+��Cl-��������Һ�п��ܺ���Na+��Cl-����B��ȷ��
C������ʵ���������ȷ���Ƿ���Al3+���������п��ܺ���Al3+����C����
D���������Ϸ�����֪��Һ�к���NH4+��Mg2+��SO42-��NO3-�������C�п��ܴ���NaNO3��NH4Cl��MgSO4����D��ȷ��
��ѡC��
���� ���⿼���˳������ӵ����ʼ����鷽������Ŀ�Ѷ��еȣ���ȷ���ʵ����ʼ����ⷴӦ�����ǽⱾ��ؼ����������ʵ��ܽ��ԡ����ʵ����ʼ������Ϣ���������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | 2H2��g��+O2��g��=2H2O ��l����H=-571.6 kJ/mol��ȼ���ȣ� | |
| B�� | NaOH��aq��+CH3COOH��aq��=CH3COONa��aq��+H2O��l����H=-57.3kJ/mol���к��ȣ� | |
| C�� | H2O ��g��=H2��g��+$\frac{1}{2}$O2��g����H=-242 kJ/mol ����Ӧ�ȣ� | |
| D�� | C��s��+O2�� g ��=CO2��g����H=-393.5 kJ/mol����Ӧ�ȣ� |
| A�� | ��״���£�22.4 L�����к���NA�����ʷ��� | |
| B�� | 1 mol Cl2�μӷ�Ӧת�Ƶ�����һ��Ϊ2NA | |
| C�� | NA��NO2��92g N2O4������ͬ����ԭ���� | |
| D�� | ��״����22.4L CO�볣����44gCO2����̼ԭ������� |
| A�� | Al��ϡH2SO4��ϡHNO3��Ӧ���������β��ų����壬˵��Al��������������û���Ӧ | |
| B�� | ������Ũ������ʹ�����������ۻ������ǿ��ڳ���������������Ʒ���ء�����Ũ���� | |
| C�� | Cl2��SO2����ʹƷ����Һ��ɫ��˵�����߶��������� | |
| D�� | ����Һ�еμ������ữ��BaCl2��Һ���ְ�ɫ������˵������Һ��һ����SO42-��SO32-�е�һ�ֻ����� |
| A�� | ��ȥ�����л��е�п�ۣ��ɼ������������������Һ������ | |
| B�� | �÷�̪��Һ��һ���Լ���ϡ���ᡢ����ʯ��ˮ�����Ȼ�����Һ | |
| C�� | Ҫ��ȥCO2�����е�����HCl���壬�ɽ���ͨ��������NaOH��Һ | |
| D�� | �����κ��Լ����ɼ���KCl��Һ��NaOH��Һ��K2SO4��Һ��CuSO4��Һ |
| A�� | ��ʯ���� | B�� | ��Ʒ��� | C�� | �����ڻ� | D�� | ʳ�︯�� |

| A�� | a��b������ʱ����Ƭ�ϻ��н���ͭ�������е������� | |
| B�� | a��b�õ�������ʱ��Cu2+�����缫�ƶ� | |
| C�� | a��b�ֱ�����ֱ����Դ��������ʱ��������������Ϊ�������������������� | |
| D�� | ����a��b�Ƿ��õ������ӣ�����K3[Fe��CN��6]��Һ����ɫ��������Һ���¶ȶ������� |
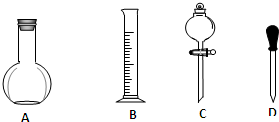 ʵ������Ҫ��NaOH��������0.1mol/LNaOH��Һ450mL����Ũ��������0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ��NaOH��������0.1mol/LNaOH��Һ450mL����Ũ��������0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮