��Ŀ����
ij��Һ���п��ܺ������������еļ��֣�K+��NO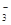 ��SO
��SO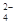 ��NH
��NH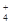 ��CO
��CO ����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
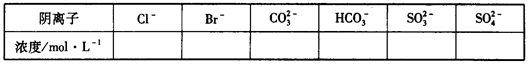
ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ672mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
��1������Һ��һ�����ڵ������� ��
��2������Һ��һ�������ڵ������� ��
��3������Һ�п��ܴ��ڵ������� ����ó��˽��۵������� ��
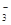 ��SO
��SO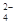 ��NH
��NH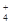 ��CO
��CO ����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺
����������Һ����ˮ�����������H+��OH������ȡ200mL����Һ����Ϊ���ȷݽ�������ʵ�飺ʵ��1����һ�ݼ����������ռ���ȣ������������ڱ�״����Ϊ672mL��
ʵ��2���ڶ����ȼ������������ᣬ�������ټ���������BaCl2��Һ���ù���2.33g��
��1������Һ��һ�����ڵ������� ��
��2������Һ��һ�������ڵ������� ��
��3������Һ�п��ܴ��ڵ������� ����ó��˽��۵������� ��
��1��NO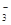 ��SO
��SO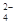 ��NH
��NH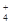 ��2��CO
��2��CO
��3��K+ ������֪����Һ�к�NH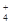 0.03mol��SO
0.03mol��SO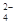 0.01mol���ݵ�����ԭ��������NO
0.01mol���ݵ�����ԭ��������NO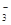 ����NO
����NO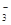 Ϊ0.01mol������K+����NO
Ϊ0.01mol������K+����NO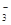 ����0.01mol������K+��
����0.01mol������K+��
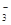 ��SO
��SO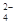 ��NH
��NH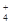 ��2��CO
��2��CO
��3��K+ ������֪����Һ�к�NH
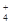 0.03mol��SO
0.03mol��SO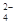 0.01mol���ݵ�����ԭ��������NO
0.01mol���ݵ�����ԭ��������NO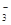 ����NO
����NO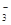 Ϊ0.01mol������K+����NO
Ϊ0.01mol������K+����NO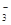 ����0.01mol������K+��
����0.01mol������K+�������������1������ʵ��1����һ�ݼ����������ռ���ȣ���NH4++OH-=NH3��+H2O���������״����Ϊ224mL���壬֤������NH4+�������ʵ���Ϊ0.01mol��
ʵ��2���ڶ����ȼ������������ᣬ��������һ��������CO32-���ټ�������BaCl2��Һ���ù���2.33g��֤��һ������SO42-�������ʵ���Ϊ��n="m/M" ="2.33g/233g/mol" =0.01mol��������Һ�еĵ���غ㣬��Һ�ʵ����ԣ���һ�����м����ӣ��Ҽ����ӵ�Ũ�ȡ�0.01mol��2?0.01mol�� 0.1L �T0.1mol/L�����Ը���Һ�п϶�����NH4+��S042-��K+��
�ʴ�Ϊ��K+��NH4+��S042-��
��2��ʵ��2���ڶ����ȼ������������ᣬ����������CO32-�������CO32-��CO32-+2H+=H2O+CO2�������ж�����̼����������ʴ�Ϊ��CO32-��
��3�����ݣ�1��֪�������ӵ�Ũ�ȡ�0.01mol��2?0.01mol ��0.1L =0.1mol/L�����K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��
�ʴ�Ϊ��NO3-��������֪��NH4+���ʵ���Ϊ0.01mol��SO42-���ʵ���Ϊ0.01mol��������Һ�ʵ�����ԭ����Ӧ�ú���K+�����K+�����ʵ�������0.01mol����NO3-�����K+�����ʵ�������0.01mol����Ӧ����NO3-��

��ϰ��ϵ�д�
�����Ŀ