��Ŀ����
����Ŀ������X��Y��Z����Ԫ�أ���֪�����������
��X��Y��Z�ĵ����ڳ����¾�Ϊ���塣
�� X�ĵ�����Z�ĵ�����ȼ�գ�����XZ��ȼ��ʱ����ʲ�ɫ��
�� XZ��������ˮ����ˮ��Һ�е����X����Z����XZ��ˮ��Һ��ʹʯ����Һ��졣
�� ������X�ĵ��ʿ���һ����Y�ĵ��ʻ�������������X2Y��X2Y������ΪҺ�塣
�� Z�ĵ�������X2Y�У�������Һ����Ư�����á�
������������⣺
(1)д��XZ��X2Y�Ļ�ѧʽ��XZ_____��X2Y______
(2)Z�ĵ�������X2Y����Һ��Ư�����õ�������______(д��ѧʽ)��
(3)д��X�ĵ�����Z�ĵ�����ȼ�յĻ�ѧ����ʽ_____________��
(4)Z�ĵ���������������Һ������Ӧ�Ļ�ѧ����ʽ��_________________��
���𰸡�HCl H2O HClO H2+Cl2![]() 2HCl Cl2��2NaOH��NaCl��NaClO��H2O
2HCl Cl2��2NaOH��NaCl��NaClO��H2O
��������
��X�ĵ�����Z�ĵ�����ȼ�գ�����XZ��ȼ��ʱ����ʲ�ɫ��XZ��������ˮ��XZ��ˮ��Һ��ʹʯ����Һ����֪XZΪHCl��XΪH2��ZΪCl2����������H2����һ����Y�ĵ��ʻ�������������H2Y��H2Y������ΪҺ���֪H2YΪH2O��
��1��XZΪHCl��X2YΪH2O���ʴ�Ϊ��HCl��H2O��
��2��Cl2����H2O������Cl2��H2O��Ӧ����HClO��HClO����ǿ������ʹ��Һ����Ư���ԣ��ʴ�Ϊ��HClO��
��3��H2��Cl2��ȼ�գ�����HCl��ȼ��ʱ����ʲ�ɫ����Ӧ�Ļ�ѧ����ʽΪH2+Cl2![]() 2HCl���ʴ�Ϊ��H2+Cl2
2HCl���ʴ�Ϊ��H2+Cl2![]() 2HCl��
2HCl��
��4��Cl2������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪCl2��2NaOH��NaCl��NaClO��H2O���ʴ�Ϊ��Cl2��2NaOH��NaCl��NaClO��H2O��

����Ŀ��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
������ | CO |
������ | Al3����Fe3����Mg2����NH��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ����(V)�Ĺ�ϵ��ͼ��ʾ��
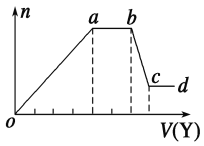
��1����Y�����ᣬ��oa��ת��Ϊ����������(ָ��Դ��X��Һ�ģ���ͬ)��___��ab�η�����Ӧ��������______________��bc�η�����Ӧ�����ӷ���ʽΪ______________��
��2����Y��NaOH��Һ����X��һ�����е�������___________________��ab�η�Ӧ�����ӷ���ʽΪ_______________________________________��