��Ŀ����
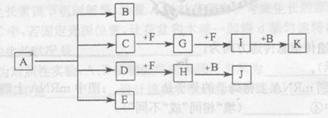
��֪������A�ǻ���ɫ���壬C�ǵ���ɫ���壬EΪ���ɫ���塣�ں��ʵķ�Ӧ�� ���£����ǿ�����ͼ���з�Ӧ��

(1) ��ͼF���ʵĻ�ѧʽ________
(2) ��Ҫд����D�Ʊ�E�����ʵ���������_______��
(3) д��B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽ_______
(4) д��B�ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��Ӧʽ��_______
(5) ��һ���¶��£�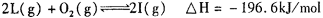 ����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������

(1) ��ͼF���ʵĻ�ѧʽ________
(2) ��Ҫд����D�Ʊ�E�����ʵ���������_______��
(3) д��B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽ_______
(4) д��B�ڳ�ʪ�Ŀ����з����绯ѧ��ʴʱ�������ĵ缫��Ӧʽ��_______
(5) ��һ���¶��£�
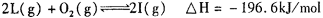 ����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=������
����һ���ݻ������ �����У�����2mol L��1molO2,ʹ֮��ַ�Ӧ���ų�������ΪQ����Q_______196. 6kJ (� >������������=��������8�֣� ��1�� Fe2O3 (1��) ��
��2�� ��FeCl3������Һ��μ��뵽��ˮ�У����ֱ����ɫ��Ϊ���ɫ��2�֣���
��3��Fe+S FeS��2�֣���
FeS��2�֣���
��4�� 2H2O+O2+4e- =4OH-��2�֣�����5��< ��1�֣�
��2�� ��FeCl3������Һ��μ��뵽��ˮ�У����ֱ����ɫ��Ϊ���ɫ��2�֣���
��3��Fe+S
 FeS��2�֣���
FeS��2�֣�����4�� 2H2O+O2+4e- =4OH-��2�֣�����5��< ��1�֣�
�����������������Ϣ������A�ǻ���ɫ���壬��AΪCl2��C�ǵ���ɫ���壬��CΪNa2O2��S��EΪ���ɫ���壬��EΪFe(OH)3��BΪFe��CΪS��DΪFeCl3��GΪFeS��FΪ��Fe2O3��LΪSO2��IΪSO3���������Ϸ�����
(1) F���ʵĻ�ѧʽΪFe2O3��
(2) ��D�Ʊ�E�����ʵ����������ǽ�FeCl3������Һ��μ��뵽��ˮ�У����ֱ����ɫ��Ϊ���ɫ��
(3) B+ C��G��һ�������·����Ļ�ѧ��Ӧ����ʽΪFe+S
 FeS��
FeS��(4) Fe��ʪ�Ŀ����з����绯ѧ��ʴ��������ʴ���Ǹ�����������O2�������ĵ缫��ӦʽΪ2H2O+O2+4e- =4OH-��
(5)��ӦΪ���淴Ӧ�����ܽ��е��ף���Q<196. 6kJ��
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע���ƶ�FeΪ������Ĺؼ����ӻ��ϼ۱仯�ĽǶȷ�����

��ϰ��ϵ�д�
�����Ŀ