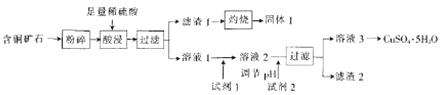
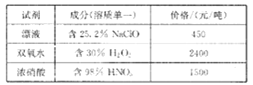
��Ŀ����
����Ŀ����������ҽҩ��ʯ����ҵ���й㷺��;����ͼ��ģ�ҵ�Ʊ��������Ʒ�����Ƶ����̡�
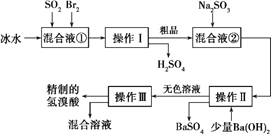
�����������̻ش���������:
��1�����Һ���з�����Ӧ�Ļ�ѧ����ʽΪ________________________��
��2�������Ƚ���γ�BrCl,BrCl��������±�ص������ơ���BrCl��ˮ������Ӧ�Ļ�ѧ����ʽΪ___________________��
��3������Ba(OH)2��Ӧ�����ӷ���ʽΪ______________�������������______��������һ�������ڷ���______����(ѡ����)
a�������Һ�� b��������� c���������ܵ�Һ�� d�����ܵ�Һ��
��4��������������ӦΪ��ɫҺ�壬��ʵ�ʹ�ҵ�������Ƶõ�������(��ҵ������)�����е����Ļ�ɫ�����Ǽס�����ͬѧ����˼�ʵ�����̽������ͬѧ���蹤ҵ������ʵ���ɫ����Ϊ����F![]() ,������֤���ü������õ��Լ�Ϊ______����Ӧ�����ӷ���ʽΪ_____________��
,������֤���ü������õ��Լ�Ϊ______����Ӧ�����ӷ���ʽΪ_____________��
���𰸡� SO2+Br2+2H2O![]() H2SO4+2HBr BrCl+H2O
H2SO4+2HBr BrCl+H2O![]() HCl+HBrO Ba2+ + SO42- == BaSO4�� ���� d KSCN��Һ Fe3+ +3SCN- == Fe(SCN)3
HCl+HBrO Ba2+ + SO42- == BaSO4�� ���� d KSCN��Һ Fe3+ +3SCN- == Fe(SCN)3
����������1��Br2����ǿ�����ԣ�����Һ�н�SO2����ΪH2SO4����������ԭΪHBr����Ӧ����ʽΪSO2+Br2+2H2O��H2SO4+2HBr����2����Cl2��H2O���ƣ�BrCl��ˮ������Ӧ�Ļ�ѧ����ʽΪBrCl+H2O��HCl+HBrO����3������Ba(OH)2��Ӧ�������ᱵ�����ӷ���ʽΪBa2++SO42-��BaSO4�����ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ��������һ�����û��ܵ�Һ��ķ��룬��ѡd����4����KSCN��Һ����Fe3+��ȡ������Һ�μ�KSCN��Һ����Һ���Ѫ��ɫ��˵��������ʵ���ɫ����Ϊ��Fe3+������ʽΪFe3+ +3SCN-��Fe(SCN)3��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ����1��Zn����ϡ���ᷴӦһ��ʱ���Ӧ���ʻ�����������Ȼ����Ũ�����Ӧ�������Լӿ졣�ɴ��жϣ�Ӱ�컯ѧ��Ӧ���ʵ�������______��______��
��2��п�����ᷴӦ���ʿ���ͨ���۲�_________�����жϣ�Ҳ��ͨ��ʵ��ⶨ��ͨ��ʵ��ⶨп�����ᷴӦ���ʣ���������Ӧʱ���⣬����Ҫ��������������_______�� _____��
��3��Ϊ̽��п�����ᷴӦ���̵����ʱ仯��ijͬѧ��ʵ��ⶨ�����ǣ���100mlϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£���������ѻ���Ϊ��״������
ʱ��/min | 1 | 2 | 3 | 4 | 5 |
���/mL | 50 | 120 | 232 | 290 | 310 |
����һʱ��η�Ӧ�������_______���0~1 min����1~2 min����2~3 min����3~4 min����4~5min������
��2~3 minʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ���ʣ�����Һ������䣩Ϊ____________��
���Է���1~3minʱ������Ӧ���ʱ�����Ҫԭ��_________________________ ��
����Ŀ������ˮ�ֱ�������и�ѡ����������Һ�У���ʵ������ó��Ľ�����ȫ��ȷ������ ��
ѡ�� | ��ˮ����������Һ�� | ʵ������ | ���� |
A | ����KSCN��FeCl2��Һ | ��� | Cl2���л�ԭ�� |
B | ���з�̪��NaOH��Һ | ��ɫ | Cl2����Ư���� |
C | ��ɫʯ����Һ | �ȱ�����ɫ | Cl2�������ԡ�Ư���� |
D | KI������Һ | ����ɫ | Cl2���������� |
A. A B. B C. C D. D