��Ŀ����
����Ŀ���밴������Ҫ��ش����⣺
��1������Ȼ�����Һ�Ļ�ѧ����ʽ��__________________________________��
��2������ˮ������ӷ���ʽ��________________________________��
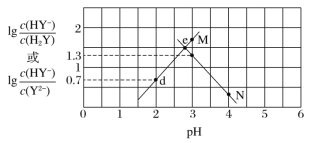
��3��0.1 mol/L ��̼������Һ������Ũ���ɴ�С˳��Ϊ��__________________________��
��4��Ũ�� Al2(SO4)3 ��Һ��Ũ��С�մ�(NaHCO3)��Һ��Ͽ���������������ӷ�Ӧ����ʽ��ʾ����ԭ��__________________________��
��5����25���� pH=12 �� Ba(OH)2 ��ҺaL�� pH=1��HCl��ҺbL ��ϣ������û��ҺΪ���ԣ��� a��b= __________________________��(��Һ����仯���Բ���)��
��6��pH=3 �� NH4Cl ��Һ����ˮ������� c(H+)= __________________________��
��7������ʱ��Fe(OH)3 ���ܶȻ����� Ksp��1��10��38��Ҫʹ��Һ�е� Fe3��������ȫ(������ c(Fe3��)<10��5 mol��L��1)���� ��Һ�� pH Ӧ����____________________________��
���𰸡�2NaCl��2H2O ![]() Cl2����2NaOH��H2�� S2-+H2O
Cl2����2NaOH��H2�� S2-+H2O![]() HS-+OH- c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+) Al3++3HCO3--=Al(OH)3��+3CO2�� 10:1 1��10-3mol/L 3
HS-+OH- c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+) Al3++3HCO3--=Al(OH)3��+3CO2�� 10:1 1��10-3mol/L 3
��������
��1���Ȼ�����Һ���������������ơ�������������
��2��S2-ˮ�⣬����HS-��OH-��
��3��̼������Һ�д���Na+��CO32-��H+��OH-������ǿ�������Σ�ˮ��Һ�ʼ��ԣ�����CO32-ˮ������HCO3-��
��4��Al2(SO4)3 ��Һ��С�մ��ϣ�Al3+��HCO3-����˫ˮ�ⷴӦ������CO2��Al(OH)3��
��5�����ҺΪ���ԣ���n(H+)=n(OH-)��
��6��NH4Cl ��ҺΪ����Һ����Һ�е�H+ȫ������ˮ���룻
��7��Ksp��c(Fe3+)c3(OH-)������c(Fe3��)<10��5 mol��L��1�����c (OH-)����һ������c (H+)��ȷ��pH�Ĵ�С��
��1���Ȼ�����Һ���������������ơ���������������Ӧ����ʽΪ��2NaCl��2H2O ![]() Cl2����2NaOH��H2����
Cl2����2NaOH��H2����
��2��S2-ˮ�⣬����HS-��OH-��ˮ�ⷽ��ʽΪ��S2-+H2O![]() HS-+OH-��
HS-+OH-��
��3��̼������Һ�д���Na+��CO32-��H+��OH-������ǿ�������Σ�ˮ��Һ�ʼ��ԣ�����CO32-ˮ������HCO3-����˸����ӵ�Ũ�ȹ�ϵΪ��c(Na+) >c(CO32-) >c(OH-) >c(HCO3-) >c(H+)��
��4��Al2(SO4)3 ��Һ��С�մ��ϣ�Al3+��HCO3-����˫ˮ�ⷴӦ������CO2��Al(OH)3������ʽΪ��Al3++3HCO3--=Al(OH)3��+3CO2����
��5�����ҺΪ���ԣ���n(H+)=n(OH-), pH=12 �� Ba(OH)2 ��ҺaL��n(OH-)=0.01a��pH=1��HCl��ҺbL��n(H+)=0.1b��0.01a=0.1b����ôa:b=10:1��
��6��NH4Cl ��ҺΪ����Һ��pH=3����Һ�е�H+ȫ������ˮ���룬��c(H+)=10-3mol/L��
��7��Ksp��c(Fe3+)c3(OH-) ����c(Fe3��)=10��5 mol��L��1����c (OH-)=![]() =10-11mol/L��c (H+)=
=10-11mol/L��c (H+)=![]() =10-3mol/L��pH=3��������� c(Fe3��)<10��5 mol��L��1������pH����3��
=10-3mol/L��pH=3��������� c(Fe3��)<10��5 mol��L��1������pH����3��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�