��Ŀ����
����Ŀ������������ȹ��ɽ������ʼ���������ҽ�������й㷺��Ӧ�á�
��1����̬ԭ�����ļ۵����Ų�ʽΪ_____��
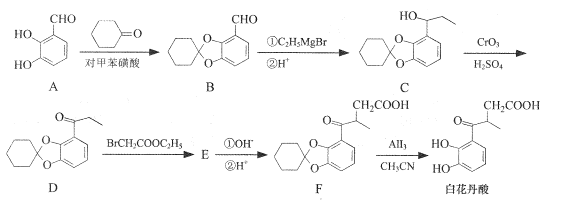
��2������ҩ��ɳ�����������Ҷ��Წ���Ľṹ��ʽ��ͼ��ʾ��
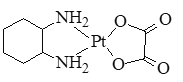
�ٷ����е�ԭ�ӹ�����ӻ�������_____��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_____��
��1 mol �Ҷ�������к��ЦҼ�����ĿΪ_____��
��3��̼����[La2(CO3)3]�������Ƹ���Ѫ֢��
��д����CO![]() ��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽ_____��
��Ϊ�ȵ������һ�ַ��ӵĻ�ѧʽ_____��
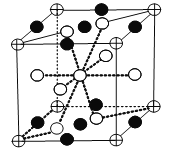
�������Ͻ�����ڴ��⣬�������Ļ�ѧʽΪLaNi5(H2)3������С�ظ��ṹ��Ԫ��ͼ��ʾ��![]() ��
��![]() ��
��![]() ���������е�������������ͼ��
���������е�������������ͼ��![]() ����������_____��
����������_____��
���𰸡� 3d84s2 sp3 N��O��C 7NA SO3 H2
����������1��Ni��ԭ������Ϊ28�������Ų�ʽΪ1s22s22p63s23p63d84s2����۵����Ų�ʽ��3d84s2����2���ٿ���ҩ��ɳ���������е�ԭ�Ӽ۵���������4��Ϊsp3�ӻ���C��N��O����ͬһ����Ԫ����ԭ���������μ�С��ͬһ����Ԫ�صĵ�һ����������ԭ������������������ڢ�A��Ĵ��ڵڢ�A��ģ��������һ�����ܴ�С˳����N��O��C���ڵ�������������˫����һ��������һ�������������1���Ҷ������һ��7��������2����������1 mol�Ҷ�������к�����������ĿΪ7NA����3���ȵ�������ָ������ͬ�۵�����Ŀ��ԭ����Ŀ�ķ��ӻ����ӣ���CO32-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪ��SO3��(4)���ݾ�̯�����㣬![]() �ĸ���Ϊ8��1/8=1��
�ĸ���Ϊ8��1/8=1��![]() �ĸ���Ϊ8��1/2+1=5����ĸ���Ϊ8��1/4+2��1/2=3�����ݻ�ѧʽLaNi5(H2)3��֪��ͼ�С����������H2��
�ĸ���Ϊ8��1/2+1=5����ĸ���Ϊ8��1/4+2��1/2=3�����ݻ�ѧʽLaNi5(H2)3��֪��ͼ�С����������H2��

 �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�