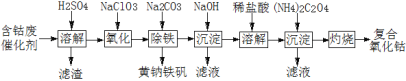
��Ŀ����
����Ŀ���������ֿ���������A��B��C������A��B���Σ�C�Ǽ��������ˮ���������������������±���ʾ��
������ | Na�� H�� Ba2�� |
������ | OH�� CO32�� SO42�� |
��������������ش����⣺
(1)C�Ļ�ѧʽΪ_____________��
(2)A��Һ��B��Һ��Ӧ����������X����X�Ļ�ѧʽΪ_______
(3)A��B��Һ��C��Һ��Ӧ�ɷֱ����ɰ�ɫ����D��E������D������ϡ����
�� B�Ļ�ѧʽΪ_____________��������ҺB�е������ӵķ�����___________________
�� D����ϡ��������ӷ���ʽΪ________________________________
�� D��E�Ļ����a g�������������ᣬ��ȫ��Ӧ���ɵ������ڱ�״�������Ϊb L��������E�ڻ�����е����������ı���ʽΪ________________________________________
���𰸡�Ba(OH)2 CO2 NaHSO4 ȡ����B��Һ���Թ��У��ȼ�������ϡ���ᣬ��Һ�������������ټ���BaCl2��Һ��������������ϡ����İ�ɫ��������˵����������ΪSO42- 2H++BaCO3=Ba2++H2O+CO2�� ![]()
��������
��1������������A��B��C��C�Ǽ��C�к���OH-��Na+��Ba2+��̼�ᱵ�����ᱵ���dz���������C��Ba(OH)2��
��2��A��B���Σ��Ҷ��ǿ������Σ�A��Һ��B��Һ��Ӧ����������X����Ӧ�����������ƺ�̼���ƣ�
��3��A��B��Һ��C��Һ��Ӧ�ɷֱ����ɰ�ɫ����D��E������D������ϡ���ᣬ��DӦ����BaCO3����A��Na2CO3��B��NaHSO4��E��BaSO4���ݴ˷������
��1��ͨ�����Ϸ�����C�������������仯ѧʽΪBa(OH)2��
�ʴ�Ϊ��Ba(OH)2��
��2��A��̼���ơ�B���������ƣ����߷�Ӧ���ɶ�����̼���壬����X��CO2��
�ʴ�Ϊ��CO2��
��3����ͨ�����Ϸ���֪��B�Ļ�ѧʽΪNaHSO4��B��������Ϊ��������ӣ�����鷽�����ȼ�ϡ�����ȥ�������Ӹ��ţ�Ȼ���ٵμ��Ȼ�����Һ������а�ɫ�������ɣ���˵��������������ӣ�
�ʴ�Ϊ��NaHSO4��ȡ����B��Һ���Թ��У��ȼ�������ϡ���ᣬ��Һ�������������ټ���BaCl2��Һ��������������ϡ����İ�ɫ��������˵����������ΪSO42-��
��D��̼�ᱵ��̼�ᱵ��ϡ���ᷴӦ�������ᱵ��������̼��ˮ�����ӷ���ʽΪ��2H++BaCO3=Ba2++H2O+CO2����
�ʴ�Ϊ��2H++BaCO3=Ba2++H2O+CO2����
��D��̼�ᱵ��E�����ᱵ��̼�ᱵ��ϡ���ᷴӦ����ȫ��Ӧ���ɵ������ڱ�״�������ΪbL�������ᱵ��ϡ�����Ӧ��n(CO2)=![]() =
=![]() mol������Cԭ���غ��n(CO2)=n(BaCO3)=
mol������Cԭ���غ��n(CO2)=n(BaCO3)=![]() mol����m(BaCO3)=
mol����m(BaCO3)=![]() mol��197g/mol=
mol��197g/mol=![]() g�����ᱵ������=ag
g�����ᱵ������=ag![]() g����E�ڻ�����е����������ı���ʽΪ
g����E�ڻ�����е����������ı���ʽΪ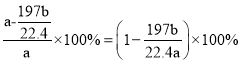 ��
��
�ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�