题目内容
【化学选修-有机化学基础】(15分)以烯烃为原料,合成某些高聚物的路线如下:
已知: (或写成
(或写成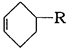 )
)
⑴CH3CH=CHCH3的名称是____________。⑵X中含有的官能团是______________。
⑶A→B的化学方程式是_____________。⑷D→E的反应类型是_______________。
⑸甲为烃,F能与NaHCO3反应产生CO2。
①下列有关说法正确的是_______________。
a.有机物Z能发生银镜反应 b.有机物Y与HOCH2CH2OH互为同系物
c.有机物Y的沸点比B低 d.有机物F能与己二胺缩聚成聚合物
②Y的同分异构体有多种,写出分子结构中含有酯基的所有同分异构体的种类数__ ___。
③Z→W的化学方程式是__________________。
⑹高聚物H的结构简式是_________________。
(1)2-丁烯;
(2)碳碳双键、氯原子; (3)ClCH2CH2CH2CH2Cl+2NaOH CH2=CH-CH=CH2+2NaCl+2H2O;
(3)ClCH2CH2CH2CH2Cl+2NaOH CH2=CH-CH=CH2+2NaCl+2H2O;
(4)消去反应;
(5)①ad;② 4③ +
+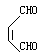 →
→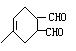
(6)
解析试题分析:(1)根据烯烃的命名CH3CH=CHCH3的名称是2-丁烯;
(2)由E的产物的结构简式倒推出E为1,3-环己二烯,B为1,3-丁二烯,所以A为1,4-二卤代烃,A是X的加氢产物,所以X中存在碳碳双键、氯原子;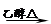 (3)A→B发生卤代烃的消去反应生成烯烃,化学方程式为
(3)A→B发生卤代烃的消去反应生成烯烃,化学方程式为 ClCH2CH2CH2CH2Cl+2NaOH CH2=CH-CH=CH2+2NaCl+2H2O;
ClCH2CH2CH2CH2Cl+2NaOH CH2=CH-CH=CH2+2NaCl+2H2O;
(4)D中有2个溴原子,E为1,3-环己二烯,所以D到E发生消去反应;
(5)①1,4-二甲苯被酸性高锰酸钾氧化生成F是对苯二甲酸,a、A中的Cl原子在碳链的端位,所以水解得Y中存在-CH2OH结构, Y发生催化氧化生成Z中存在醛基,能发生银镜反应,正确;b、Y中含有2个羟基和1个碳碳双键,与HOCH2CH2OH不是同系物,错误;c、Y是含碳碳双键的醇类,B为烯烃,所以Y的沸点高,错误;d、有机物F是对苯二甲酸,能与己二胺缩聚成聚合物多肽或蛋白质,正确,答案选ad;
②Y中含有2个羟基和1个碳碳双键,所以Y的含有酯基的所有同分异构体共4种,分别是甲酸丙酯、甲酸异丙酯、乙酸乙酯、丙酸甲酯;
③由以上分析知Z是 ,根据W的加氢产物判断甲为
,根据W的加氢产物判断甲为 ,所以Z→W发生类似已知的反应,化学方程式是
,所以Z→W发生类似已知的反应,化学方程式是 +
+ →
→
(6)H是对二苯甲酸与W的加氢产物发生缩聚反应的产物,所以H的结构简式为
考点:考查有机推断,有机物的命名,官能团的判断,反应类型的判断,化学方程式、结构简式的书写,同分异构体的判断

 阅读快车系列答案
阅读快车系列答案(16分)Favorskii反应是化工生产中的重要反应,它是利用炔烃与羰基化合物在强碱性下发生反应,得到炔醇,反应原理为:
已知:
以下合成路线是某化工厂生产流程的一部分:
请回答下列问题:
(1)写出F中官能团的名称 。
(2)写出D的名称(系统命名) 。
(3)④的反应类型是 ;B的结构简式是 。
(4)写出反应⑥的化学方程式为 。
(5)H是D的同分异构体,核磁共振氢谱有3种峰且属于炔烃的结构简式为
、 。
(6)有关C的说法正确的是
| A.能使溴的四氯化碳溶液褪色 | B.能和氢氧化钠溶液反应 |
| C.能使酸性高锰酸钾褪色 | D.能与乙酸发生酯化反应 |





 F为加成反应,化合物X的结构简式为 。
F为加成反应,化合物X的结构简式为 。 化合物
化合物 是合成抗病毒药阿昔洛韦的中间体,请设计合理方案以
是合成抗病毒药阿昔洛韦的中间体,请设计合理方案以 和
和 为原料合成该化合物(用合成路线流程图表示,并注明反应条件)。合成路线流程图示例如下:
为原料合成该化合物(用合成路线流程图表示,并注明反应条件)。合成路线流程图示例如下:



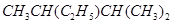 的名称是 。
的名称是 。