��Ŀ����
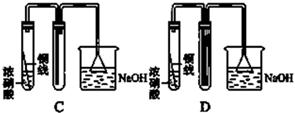
ij��ѧѧϰС��ͬѧ����ʵ�������е���ȡ������ҩƷ���������ͼ��ʾ��ʵ��װ�ã����ּг�����δ����������ȡ��̽�������Ļ�ԭ�ԡ����鷴Ӧ�����ش��������⣺

��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�м�ʯ�ҵ������� ��
��3��C�к�ɫ�����죬�Ҳ���������Կ�������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
D�����������________________________________________________________________________��
��4����װ�ô�������ȱ�ݣ���ȱ���� ��
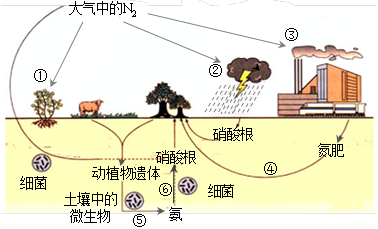
��5����ҵ�г��õ����������ڸ��¡���ѹ������ý�������������ºϳɰ�������С��ͬѧģ�������Ҳ�ϳɳ��˰�������֪��ʼʱ����2 mol N2��6 mol H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ������5 min�������������ʵ���������1 mol���������ʱ������H2��ʾ�Ļ�ѧ��Ӧ����
Ϊ ��

��1��A�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��B�м�ʯ�ҵ������� ��
��3��C�к�ɫ�����죬�Ҳ���������Կ�������Ⱦ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
D�����������________________________________________________________________________��
��4����װ�ô�������ȱ�ݣ���ȱ���� ��
��5����ҵ�г��õ����������ڸ��¡���ѹ������ý�������������ºϳɰ�������С��ͬѧģ�������Ҳ�ϳɳ��˰�������֪��ʼʱ����2 mol N2��6 mol H2����һ���ݻ�Ϊ2 L���ܱ������з�����Ӧ������5 min�������������ʵ���������1 mol���������ʱ������H2��ʾ�Ļ�ѧ��Ӧ����
Ϊ ��
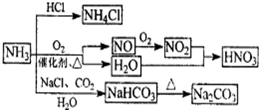
��1��Ca(OH)2��2NH4Cl CaCl2��2NH3����2H2O��2�֣���
CaCl2��2NH3����2H2O��2�֣���
��2�����ﰱ����1�֣���
��3��3CuO+2NH3 3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣���
3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣���
��4��ȱ��β������װ�ã�1�֣���
��5��0.15 mol/(L��min)��2�֣�
 CaCl2��2NH3����2H2O��2�֣���
CaCl2��2NH3����2H2O��2�֣�����2�����ﰱ����1�֣���
��3��3CuO+2NH3
 3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣���
3Cu+N2+3H2O��2�֣�����ˮ����ͭ������1�֣�����4��ȱ��β������װ�ã�1�֣���
��5��0.15 mol/(L��min)��2�֣�
�����������1��װ��A���Ʊ������ģ�������з�����Ӧ�Ļ�ѧ����ʽ��Ca(OH)2��2NH4Cl
 CaCl2��2NH3����2H2O��
CaCl2��2NH3����2H2O����2���������ɵİ����л���ˮ����������ź���ʵ���в���ˮ�IJⶨ����ʯ���Ǹ����������ˮ���������Լ�ʯ�ҵ������Ǹ��ﰱ������ֹ���Ų���ˮ�IJⶨ��
��3����ɫCuO��Ϊ��ɫ��������������ͭ��ͬʱ����һ����ɫ���壬����������Ⱦ����˸������ǵ���������ԭ���غ��֪������ˮ���ɣ���Ӧ�Ļ�ѧ����ʽΪ3CuO+2NH3
 3Cu+N2+3H2O����ˮ����ͭ����ˮ�ɰ������Ϊ��ɫ������D�е�ʵ�������ǰ�ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��
3Cu+N2+3H2O����ˮ����ͭ����ˮ�ɰ������Ϊ��ɫ������D�е�ʵ�������ǰ�ɫ��ˮCuSO4��ĩ��Ϊ��ɫ����4�������Ǵ̼������壬���Բ��������ŷŵ������У�Ӧ��β������װ�ã�������ͼ��ʾװ�����հ���
 ��
����5���ϳɰ���Ӧ�Ļ�ѧ����ʽΪ��
3H2 + N2
 2NH3 ��n�L
2NH3 ��n�L3mol 1mol 2mol 2mol
n(H2) 1mol
���n(H2)��
 ��1.5mol
��1.5mol������������Ũ����1.5mol��2L��0.75mol/L
��������ʱ������H2��ʾ�Ļ�ѧ��Ӧ���ʣ�0.75mol/L��5min��0.15 mol/(L��min)

��ϰ��ϵ�д�
�����Ŀ
 NaHSO4+HNO3�������������ΪHNO3
NaHSO4+HNO3�������������ΪHNO3

 2NO
2NO CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O