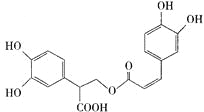
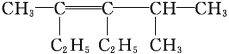
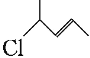
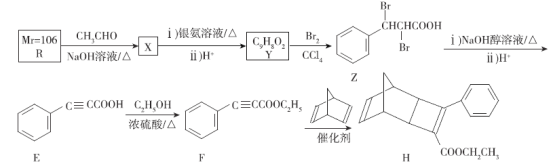
��Ŀ����
����Ŀ�������ṩ�����ƶ�Ԫ�أ�����Ҫ����գ�
��1��ԭ�Ӻ�����3�����Ӳ㣬������������Ϊ7����������ﻯѧʽ________������������Ӧˮ���ﻯѧʽ______��������������Ӧˮ������NaOH��Ӧ�Ļ�ѧ����ʽΪ_________��
��2����������Ԫ�أ�����������������Ӳ�����ͬ����Ԫ�����������Ļ�ѧʽΪ ________������������Ӧˮ�������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ_______��
��3��ԭ���������ε�����ͬ����4��Ԫ�أ������⻯��������������������Ar��ͬ����Щ�⻯��Ļ�ѧʽ�ֱ�Ϊ____________��__________��_________��______��
��4��ijԪ�ص��������������۵Ĵ�����Ϊ4��������������к���Ԫ����������Ϊ60%�����Ԫ�����������Ļ�ѧʽΪ__________������������________�����������������������������
���𰸡�Cl2O7 HClO4 HClO4+NaOH=NaClO4+H2O Al2O3 Al(OH)3+NaOH=NaAlO2+2H2O SiH4 PH3 H2S HCl SO3 ����
��������
��1��ԭ�Ӻ�����3�����Ӳ㣬������������Ϊ7�����Ԫ��ΪCl�����Ϊ+7�ۣ���������ﻯѧʽΪCl2O7������������Ӧˮ���ﻯѧʽΪHClO4��HClO4��NaOH��Ӧ�Ļ�ѧ����ʽΪHClO4+NaOH=NaClO4+H2O��
��2����������Ԫ�أ�����������������Ӳ�����ͬ�����Ԫ��ΪAl�������Ϊ+3�ۣ����������Ļ�ѧʽΪAl2O3������������Ӧˮ������Al(OH)3�������������Ʒ�Ӧ�Ļ�ѧ����ʽΪAl(OH)3+NaOH=NaAlO2+2H2O��
��3��ԭ���������ε�����ͬ����4��Ԫ�أ������⻯��������������������Ar��ͬ������4��Ԫ��λ�ڵ������ڣ��ֱ�ΪSi��P��S��Cl���γɵ��⻯��Ļ�ѧʽ�ֱ�ΪSiH4��PH3��H2S��HCl��
��4��ijԪ�ص��������������۵Ĵ�����Ϊ4�����Ԫ�ص����Ϊ+6�������Ϊ-2���������������Ļ�ѧʽΪRO3����Է�������ΪM�����ݺ���Ԫ����������Ϊ60%����![]() �������M=80�����Ԫ�ص����ԭ������Ϊ80-48=32����Ԫ��Ϊ��Ԫ�أ����������Ļ�ѧʽΪSO3���������������ۻ����
�������M=80�����Ԫ�ص����ԭ������Ϊ80-48=32����Ԫ��Ϊ��Ԫ�أ����������Ļ�ѧʽΪSO3���������������ۻ����

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�