��Ŀ����
ij�о�С�������NH3�йص�ϵ��ʵ�顣
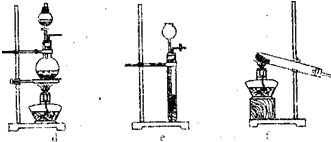
(1)����ͼ����ѡ����������������ȡ����NH3��װ�ü�ͼ(���ӱ�Ҫ�����ӡ��������ܡ���Ƥ��,�̶�װ�ú�β������װ�ò��û�)�������������Լ���

(2)��NH3ͨ����ˮ�У���N2���ɣ���Ӧ�Ļ�ѧ����ʽΪ____________________��

(3)Ϊ��֤��ͬ��ͬѹ�£���ͬ������κ����嶼������ͬ��Ŀ�ķ��ӡ�����С��ͬѧ���������ͼ��ʾ��װ�ã�ͼ��B�ܵ��ݻ���A�ܵ�2��������K1��K2��K3��K4��K5���ر�(�̶�װ�ú�β������װ���ԣ���HCl��NH3��������ʯ���ͣ�Ҳ����֮��Ӧ��װ�������Ժá�
����A���г��������ѹǿ��ȵĸ���HCl���塣������________________�����ƻ���K4��K5������C��ʹB�ܳ�����A��ͬѹ�ĸ���NH3��
�ڻ�������K3��A���е�������___________________��Ҫ�ﵽʵ��Ŀ�ģ�����Ӧ��ɲ��ָ�������ʱ��B����Ԥ�ڵ�������____________________�����۲첻��Ԥ��������Ҫԭ����____________________________________��
(1)
(2)2NH3+3Br2====6HBr+N2
(3)�ٴ�K1��K2����K2��ͨ������HCl���壬ֱ��K1���а������֣��ر�K1��K2 �����ɴ����İ��� B���г���ʯ���� ʵ��ʱA����δȫ����������HCl����
����:
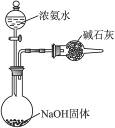
������Ҫ�����й�������ѡ����װ�����ʵ����������������������ݡ�(1)��ϸ�۲������������ѷ���û�оƾ��ƣ����������÷�Ӧ2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O���Ʊ�NH3�����������������ص㣬���NH3+H2O
CaCl2+2NH3��+2H2O���Ʊ�NH3�����������������ص㣬���NH3+H2O![]() NH3��H2O
NH3��H2O![]()
![]() +OH-���ѶԱ�С���������
+OH-���ѶԱ�С���������
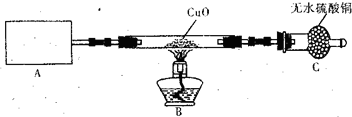
(2)NH3��N2���ϼ����ߣ���Br2�ڷ�Ӧ�бض����ϼ۽�������Br-������������ԭ��Ӧ����ʽ����ƽ����������д�����л�ѧ��Ӧ����ʽ��2NH3+3Br2====6HBr+N2����NH3����ʱ��Ȼ������Ӧ��8NH3+3Br2====N2+6NH4Br��
(3)Ҫ��A�г��������ѹǿ��ȵ�HCl���壬����A�������ͨ�������³��룬���(HCl)����(����)�������K1����K2ͨ����K1����HCl�ݳ�(�а�������)����������K3����NH3����A�ܲ�������ӦNH3+HCl====NH4Cl���������̣�ѹǿ��С��ʯ���ͳ���B��(ʣ���NH3ǡ�ó���A��)�����۲첻�����������������HCl�л��п�����ʯ�����˵�Һ�治��ƽ���¡�

��ѧ��Ӧ�����뻯ѧƽ�����ճ������ũҵ�����Ϳ�ѧ�о��о�����Ҫ�����壬��
��ѧʵ�����漰�ĵ���ƽ��Ҳ���ڻ�ѧƽ�⡣��ش��������⣺
��1��ij�о���ѧϰС��������Ϸ��ֽ���������AҲ�ܴ�����صķֽ⣬��A�Ͷ�
�����̵���Ѵ��¶Ⱦ�Ϊ500�����ҡ����Ƕ�A�Ͷ������̵Ĵ����ܽ�����
��������ʵ�顣ʵ��ʱ��������500 mL����Ϊ(��������Ӱ��ʵ������ؾ���
����)��
��һ ��MnO2������
| ʵ����� | KClO3����/g | MnO2����/g | ��Ӧ�¶�/�� | �������� |
| 1 | 8.00 | 2.00 | 500 | |
| 2 | 8.00 | 2.00 | 500 |
���� ��A������
| ʵ����� | KClO3����/g | A������/g | ��Ӧ�¶�/�� | �������� |
| 1 | 8.00 | 2.00 | 500 | |
| 2 | 8.00 | 2.00 | 500 |
��ش�����ʵ���еĴ�������Ӧ�� ��
��ɴ��о�������������һƪ�о����棬������������һ�о�����ı��⣺
��
��2����ˮ��һ�����ijͬѧȡ0.1mo/L�İ�ˮ����pH��ֽ����pH�����������Һ��
pHԼΪ11���Դ˵ó���ˮΪ����Ľ��ۡ���ͬѧ��pH��ֽ�ⶨ��ˮpH�ľ����
���� ��
��3��֤����ˮ������ij��÷����������֣�һ���跨֤��NH![]() ��ˮ�⣬һ���跨ʹ��ˮ
��ˮ�⣬һ���跨ʹ��ˮ
����ƽ�ⷢ���ƶ���
����һ��ȡ����NH4Cl��������ˮ������ʯ����Һ����Һ��죬�ɼ���Һ�����ԡ�
��ԭ���� ��
��������ȡ������ˮ�������̪�����ټ��� ������ɫ��dz��c(OH��)�½���˵����ˮ�ĵ���ƽ���� �ƶ���