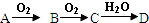��Ŀ����
�� I��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֣�
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��
��1����Y�����ᣬ����Һ�к��еĽ�����������______��ab�η�����Ӧ�������ӷ���ʽΪ______��Oa���ϱ��вμӷ�Ӧ�����ӵ����ʵ���֮��Ϊ��______[Ҫ�������ӷ��ţ���n��Na+��]��
��2����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ______�������������ӵ�ˮ�����أ�����H+��OH-Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ______[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
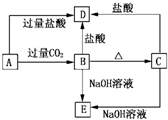
�� II����Ϊ��IVA��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ���Sn4++Sn�T2Sn2+����2Sn2++O2+4H+�T2Sn4++2H2O����2H++SnO22-?Sn��OH��2?Sn2++2OH-���Իش�
��1�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��______��______��
��2������1������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ��������ǣ�����ʽ��______��
��3��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü���______��
| ������ | CO32-��SiO32-��AlO2-��Cl- |
| ������ | Al3+��Cu2+��Mg2+��NH4+��Na+ |
��1����Y�����ᣬ����Һ�к��еĽ�����������______��ab�η�����Ӧ�������ӷ���ʽΪ______��Oa���ϱ��вμӷ�Ӧ�����ӵ����ʵ���֮��Ϊ��______[Ҫ�������ӷ��ţ���n��Na+��]��
��2����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ______�������������ӵ�ˮ�����أ�����H+��OH-Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ______[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
�� II����Ϊ��IVA��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ���Sn4++Sn�T2Sn2+����2Sn2++O2+4H+�T2Sn4++2H2O����2H++SnO22-?Sn��OH��2?Sn2++2OH-���Իش�
��1�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��______��______��
��2������1������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ��������ǣ�����ʽ��______��
��3��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü���______��

��Һ��ɫ˵����Һ�в���ͭ���ӣ�
��1�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�ͨ�����Ϸ���֪����Һ�к��е��������������ӣ�ab�η�����Ӧ��̼������Ӻ������ӷ�Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪ��CO32-+2H+=H2O+CO2��������ͼ��֪���������������ᷴӦ��Ҫ1V���ᣬƫ��������Ӻ�������Ӻ����ᷴӦ��Ҫ����4V���ᣬ�йط�Ӧ����ʽΪ��AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ����Al��OH��3+3H+=Al3++3H2O�����ݷ���ʽ֪��SiO32-����H2SiO3��Ҫ����������AlO2-����Al��OH��3��Ҫ��������֮��
=11��1������AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ��֪����n��SiO32-����n��AlO2-��=
��
=11��2��
�ʴ�Ϊ��Na+��CO32-+2H+=H2O+CO2����n��SiO32-����n��AlO2-��=11��2��
��2����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
Y��NaOH��Һ����bc���������������������Ʒ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��笠�������Ҫ�������Ƶ������2V�������������������Ʒ�Ӧ��Ҫ�������Ƶ������1V������������������Ҫ�������Ƶ������3V������������þ��Ҫ�������Ƶ������1V����n��Al3+����n��Mg2+����n��NH4+��=1��
��2=2��1��4����Һ��������������������֪��n��Al3+����n��Mg2+ ����n��NH4+����n��Cl- ��=2��1��4��12����N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
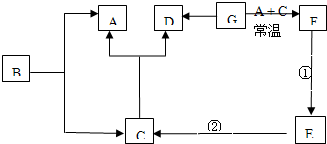
��II����1���������ᷴӦ���ɶ��Ȼ��������Ȼ��������������������Ȼ�������Ӧ����ʽΪ��Sn+2HCl=SnCl2+H2����SnCl2+Cl2=SnCl4��
�ʴ�Ϊ��Sn+2HCl=SnCl2+H2����SnCl2+Cl2=SnCl4
��2�����Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ��������������������õ��Ĺ�����SnO2���ʴ�Ϊ��SnO2��
��3�����������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ��������������ΪNH3?H2O���ʴ�Ϊ��NH3?H2O��
��1�����Y�����ᣬ����Һ�м����ᣬ�����ɳ�������a-b��ʱ�������������仯�������̼������ӷ�Ӧ�������壬����Һ�в���þ���ӡ������ӣ���b-c��ʱ�������������٣����ֳ��������ᷴӦ�����ֳ����������Ӧ��˵����Һ���й�������Ӻ�ƫ��������ӣ���������Ӻ�笠�������˫ˮ�⣬������Һ�к��е��������������ӣ�ͨ�����Ϸ���֪����Һ�к��е��������������ӣ�ab�η�����Ӧ��̼������Ӻ������ӷ�Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪ��CO32-+2H+=H2O+CO2��������ͼ��֪���������������ᷴӦ��Ҫ1V���ᣬƫ��������Ӻ�������Ӻ����ᷴӦ��Ҫ����4V���ᣬ�йط�Ӧ����ʽΪ��AlO2-+H++H2O=Al��OH��3����SiO32-+2H+=H2SiO3 ����Al��OH��3+3H+=Al3++3H2O�����ݷ���ʽ֪��SiO32-����H2SiO3��Ҫ����������AlO2-����Al��OH��3��Ҫ��������֮��
(4-
| ||
|
4-
| ||
| 2 |
| 1 |
| 3 |
�ʴ�Ϊ��Na+��CO32-+2H+=H2O+CO2����n��SiO32-����n��AlO2-��=11��2��
��2����Y���������ƣ�����Һ�м�����������Һ�������ɳ�������a-b��ʱ�������������仯���������ƺ�笠����ӷ�Ӧ�������壻��b-c��ʱ�������������٣����ֳ������������Ʒ�Ӧ�����ֳ�������Ӧ��˵����Һ�����������Ӻ�þ���ӣ�����Һ�в�����������ӡ�̼������Ӻ�ƫ��������ӣ�������Һ�к��е��������������ӣ�
Y��NaOH��Һ����bc���������������������Ʒ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��笠�������Ҫ�������Ƶ������2V�������������������Ʒ�Ӧ��Ҫ�������Ƶ������1V������������������Ҫ�������Ƶ������3V������������þ��Ҫ�������Ƶ������1V����n��Al3+����n��Mg2+����n��NH4+��=1��
| 1 |
| 2 |
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��N��Al3+����N��Mg2+ ����N��NH4+����N��Cl- ��=2��1��4��12��
��II����1���������ᷴӦ���ɶ��Ȼ��������Ȼ��������������������Ȼ�������Ӧ����ʽΪ��Sn+2HCl=SnCl2+H2����SnCl2+Cl2=SnCl4��
�ʴ�Ϊ��Sn+2HCl=SnCl2+H2����SnCl2+Cl2=SnCl4
��2�����Ȼ�����Һ�������ù��������ڸ���������ݶ��ߵ�������֪�����Ȼ�����Һ���ɵõ��������������������õ��Ĺ�����SnO2���ʴ�Ϊ��SnO2��
��3�����������Ϣ֪��Sn��OH��2�������ԣ��ܺ�ǿ�Ӧ��������ȡ������ʱӦ��������������ΪNH3?H2O���ʴ�Ϊ��NH3?H2O��

��ϰ��ϵ�д�
�����Ŀ