��Ŀ����
����Ŀ��(1)���з����п���֤�� 2HI(g) ![]() H2(g)+I2(g)�Ѵ�ƽ��״̬����_________��
H2(g)+I2(g)�Ѵ�ƽ��״̬����_________��
�ٵ�λʱ�������� n mol H2 ��ͬʱ���� n mol HI��
��һ�� H��H �����ѵ�ͬʱ��һ�� H��I�����ѣ�
�۰ٷ���ɦ�(HI)=��(I2)��
�ܷ�Ӧ����v(H2)=v(I2)= ![]() v(HI)ʱ��
v(HI)ʱ��
��c(HI):c(H2):c(I2)=2:1:1 ʱ��
���¶Ⱥ����һ��ʱ��ijһ������Ũ�Ȳ��ٱ仯��
���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯��
������һ������������ƽ����Է����������ٱ仯��
���¶Ⱥ����һ��ʱ������������ɫ���ٱ仯��
���¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯��
(2)���ںϳɰ�������˵���ﵽƽ��״̬����_________��
���𰸡��ޢ� �ޢߢ��
��������
2HI(g) ![]() H2(g)+I2(g)Ϊ�������ʵ�������ķ�Ӧ��N2 + 3H2
H2(g)+I2(g)Ϊ�������ʵ�������ķ�Ӧ��N2 + 3H2 ![]() 2NH3Ϊ�������ʵ�����С�ķ�Ӧ�����ݻ�ѧƽ��״̬�������ͱ�־�����жϡ�
2NH3Ϊ�������ʵ�����С�ķ�Ӧ�����ݻ�ѧƽ��״̬�������ͱ�־�����жϡ�
(1)2HI(g) ![]() H2(g)+I2(g)Ϊ�������ʵ�������ķ�Ӧ��
H2(g)+I2(g)Ϊ�������ʵ�������ķ�Ӧ��
�ٵ�λʱ��������n mol H2��ͬʱ������n mol HI������֮�Ȳ��������ʵ���֮�ȣ����淴Ӧ���ʲ��ȣ�����ƽ��״̬���ʢٴ���
��һ��H-H�����ѵ�Ч������H-I���γɣ�ͬʱ��һ��H-I�����ѣ����淴Ӧ���ʲ��ȣ�����ƽ��״̬���ʢڴ���
�۰ٷ����w(HI)=w(I2)������˵����ɲ��䣬����˵����ƽ��״̬���ʢ۴���
�ܷ�Ӧ����v(H2)=v(I2)=0.5v(HI)������˵�����淴Ӧ������ȣ�����˵����ƽ��״̬���ʢܴ���
��c(HI)��c(H2)��c(I2)=2��1��1������˵��Ũ���Ƿ䣬����˵����ƽ��״̬���ʢݴ���
���¶Ⱥ����һ��ʱ��������Ũ�Ȳ��ٱ仯��˵�����淴Ӧ������ȣ���ƽ��״̬���ʢ���ȷ��
���¶Ⱥ����һ��ʱ������ѹǿʼ�ղ��䣬������ѹǿ���ٱ仯������˵����ƽ��״̬���ʢߴ���
������һ�������������������䣬���ʵ������䣬ƽ����Է�������ʼ�ղ��䣬��������ƽ����Է����������ٱ仯������˵����ƽ��״̬���ʢ����
���¶Ⱥ����һ��ʱ������������ɫ���ٱ仯��˵����������Ũ�Ȳ��䣬˵��Ϊƽ��״̬���ʢ���ȷ��
���¶Ⱥ�ѹǿһ��ʱ�����������������䣬�����������䣬���������ܶ�һֱ���䣬���������ܶȲ��ٱ仯������˵����ƽ��״̬���ʢ����
�Ѵ�ƽ��״̬���Ǣޢ�ʴ�Ϊ���ޢ
(2)N2 + 3H2 ![]() 2NH3Ϊ�������ʵ�����С�ķ�Ӧ��
2NH3Ϊ�������ʵ�����С�ķ�Ӧ��
�ٵ�λʱ��������n mol H2��ͬʱ������n mol NH3������֮�Ȳ��������ʵ���֮�ȣ����淴Ӧ���ʲ��ȣ�����ƽ��״̬���ʢٴ���
��3��H-H�����ѵ�Ч��6��H-N���γɣ�ͬʱ��һ��H-N�����ѣ����淴Ӧ���ʲ��ȣ�����ƽ��״̬���ʢڴ���
�۰ٷ����w(NH3)=w(H2)������˵����ɲ��䣬����˵����ƽ��״̬���ʢ۴���
������֮�ȵ��ڻ�ѧ������֮�ȣ�����˵�����淴Ӧ������ȣ�����˵����ƽ��״̬���ʢܴ���
��Ũ��֮�ȵ��ڻ�ѧ������֮�ȣ�����˵��Ũ���Ƿ䣬����˵����ƽ��״̬���ʢݴ���
���¶Ⱥ����һ��ʱ��������Ũ�Ȳ��ٱ仯��˵�����淴Ӧ������ȣ���ƽ��״̬���ʢ���ȷ��
���¶Ⱥ����һ��ʱ����������ʵ�����С�����������������Ϊ������������ѹǿ���ٱ仯��˵����ƽ��״̬���ʢ���ȷ��
������һ�������������������䣬���ʵ�����С��ƽ����Է�����������������ƽ����Է����������ٱ仯��˵����ƽ��״̬���ʢ���ȷ��
���¶Ⱥ����һ��ʱ������������ɫʼ��Ϊ��ɫ������˵��Ϊƽ��״̬���ʢ����
���¶Ⱥ�ѹǿһ��ʱ�����������������䣬����������С�����������ܶ������������ܶȲ��ٱ仯��˵����ƽ��״̬���ʢ���ȷ��
�Ѵ�ƽ��״̬���Ǣޢߢ�⣻�ʴ�Ϊ���ޢߢ�⡣

����Ŀ������ˮ��ɽ���ǽ�ɽ��ɽ��������������������Ⱦ���е�NOx��SO2�������Ϊ���ǹ�ע����Ҫ����֮һ��
(1)SO2���ŷ���Ҫ������ú��ȼ�գ���ҵ�ϳ��ð�ˮ���շ�����β���е�SO2��
��֪���չ�������ط�Ӧ���Ȼ�ѧ����ʽ���£�
��SO2(g)+NH3��H2O(aq)= NH4HSO3(aq) ��H1= a kJ/mol��
��NH3��H2O(aq)+ NH4HSO3(aq)=(NH4)2SO3(ag)+H2O(l) ��H2= b kJ/mol��
��2(NH4)2SO3(aq)+O2(g)=2(NH4)2SO4(aq) ��H3= c kJ/mol��
��Ӧ2SO2(g)+ 4NH3��H2O(aq)+ O2(g) = 2(NH4)2SO4(aq)+ 2H2O(l)����H=______kJ/mol��
(2)ȼú���糧�����÷�Ӧ2CaCO3(s)+2SO2(g)+O2(g)![]() 2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
2CaSO4(s)+2CO2(g) ��H =681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪTKʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
ʱ��/min Ũ��/mol��L1 | 0 | 10 | 20 | 30 | 40 | 50 |
O2 | 1.00 | 0.79 | 0.60 | 0.60 | 0.64 | 0.64 |
CO2 | 0 | 0.42 | 0.80 | 0.80 | 0.88 | 0.88 |
��0��20 min�ڣ�ƽ����Ӧ����v(SO2)=_____mol/(L��min)��
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⡣�����ϱ��е������жϣ��ı������������_____������ĸ����
A��ͨ��һ������O2 B������һ�����ķ�״̼���
C���ʵ���С��������� D�������Ч�Ĵ���
(3)NOx���ŷ���Ҫ����������β�����������÷�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO����������
N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO����������
����֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ��ʾ��
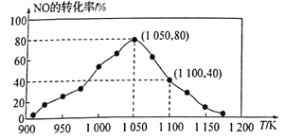
��ͼ��֪��1050Kǰ��Ӧ��NO��ת�������¶����{��������ԭ��Ϊ________________________________��
����ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp������1050K��1.1��105Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=_____________________ [��֪�������ѹ(P��)=������ѹ(P)���������]��
(4)��������Ҳ����������[(NH2)2CO]ˮ��Һ���ա�������[(NH2)2CO]ˮ��Һ���������Ϊ1��1��NO��NO2��������ɽ�NԪ��ת��Ϊ�Ի����������塣�÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
(5)����β�����ջ������÷�Ӧ2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��= k����c2(NO)��c2(CO)��v��= k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
N2(g)+2CO2(g) ��H=746.8 kJ/mol��ʵ���ã�v��= k����c2(NO)��c2(CO)��v��= k����c(N2)��c2(CO2)��k����k��Ϊ���ʳ�����ֻ���¶��йأ���
�ٴﵽƽ��������¶ȣ�k������ı���_________������>����<������=����k������ı�����
������1L�ĺ����ܱ������г���1 molCO��1 mol NO����һ���¶��´ﵽƽ��ʱ��CO��ת����Ϊ80%����k���Uk��=_____L/mol��
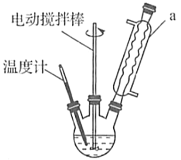
����Ŀ����������һ����Ҫ�Ļ���ԭ�ϡ�ʵ���Һϳɱ������ԭ�����й����ݼ�װ��ʾ��ͼ����:
���� | ��״ | �۵�(��) | �е�(��) | �ܶ�(g/mL) | �ܽ��� | |
ˮ | �Ҵ� | |||||
�ױ� | ��ɫҺ����ȼ�ӷ� | -95 | 110.6 | 0.8669 | ���� | ���� |
������ | ��ɫƬ״����״���� | 112.4(100����������) | 248 | 1.2659 | �� | ���� |
��������ˮ�е��ܽ�������
�¶�/�� | 4 | 18 | 75 |
�ܽ��/g | 0.2 | 0.3 | 2.2 |
ijѧϰС����ʵ�����Ʊ������롢�ᴿ�����ᣬ���ⶨ������Ʒ�Ĵ��ȣ���������:
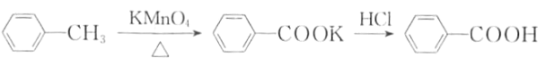
һ���Ʊ�������
��b�м���2.7mL�ױ���100mL
���������ᴿ
�ڷ�Ӧ������м���һ��������(H2C2O4)��ַ�Ӧ�����ˡ�ϴ�ӣ�����Һ���ڱ�ˮԡ����ȴ��Ȼ����Ũ�����ữ��������ȫ���������ѹ���ˣ�����������������ˮϴ�ӣ���ѹȥˮ�ֺ���ڷ�ˮԡ�ϸ���õ��ֲ�Ʒ��
�����ⶨ����
��ȡm g��Ʒ�����100mL�Ҵ���Һ����ȡ25.00mL��Һ����ƿ�У��μ�2~3�η�̪��Ȼ���ñ�Ũ�ȵ�KOH��Һ�ζ���
��ش���������:
(1)װ��b��������__________________��װ��a������Ϊ_____________________________��
(2)�����ᴿ�����м���IJ�����һ�ֶ�Ԫ���ᣬ��Ӧ����������ʽ�Ρ���ɫ����ͺ�ɫ�������ɡ���������������_________________________���������ӷ���ʽ��ʾ��Ӧ��ԭ��______________________________��
(3)��Ʒ��ѹ����ʱ����ˮϴ�ӵ�ԭ����__________________________________________��
(4)ѡ������__________���������Խ��ֲ�Ʒ��һ���ᴿ��(ѡ����ĸ)
A������ˮ����� B�������Ҵ������� C���üױ���ȡ���Һ D������
(5)�ⶨ���Ȳ����У��жϵζ��յ�ı�־��________________________________________________����m=1.200g���ζ�ʱ��ȥ0.1200mol�� L��1��KOH��Һ18.00mL�������ò�Ʒ�б��������������Ϊ__________(������λ��Ч����)��
����Ŀ��ʵ����̽���Ʊ�������ص���ɫ����������ʵ���������£�
��֪��![]() ˮ��Һ��ī��ɫ�������ԡ����Ժ������Ի����£�
ˮ��Һ��ī��ɫ�������ԡ����Ժ������Ի����£�![]() �ᷢ������������ԭ���绯����Ӧ������
�ᷢ������������ԭ���绯����Ӧ������![]() ��
��![]() �������Լ��۵㡢�ֽ��¶ȼ��±���
�������Լ��۵㡢�ֽ��¶ȼ��±���
���� |
|
|
|
|
|
�۵�/ | 406 | 368 | ���� | ���� | ���� |
�ֽ��¶�/ | 1323 |
| 530 | 190 | 240 |
(1)��ǿ���������£�![]() ��
��![]() ���ۿ��Ƶ�
���ۿ��Ƶ�![]() ���仯ѧ����ʽΪ________��Ͷ�ϵ�˳��Ϊ�ȼ���
���仯ѧ����ʽΪ________��Ͷ�ϵ�˳��Ϊ�ȼ���![]() ��
��![]() ��Ͼ��ȣ���С����ȫ���ڣ��ټ���
��Ͼ��ȣ���С����ȫ���ڣ��ټ���![]() ��Ѹ�ٽ��衣�����Ƚ�
��Ѹ�ٽ��衣�����Ƚ�![]() ��
��![]() ��ϵ�ԭ����________��
��ϵ�ԭ����________��
(2)�ܽ��������Һת������ƿ�У����ȵ���![]() �����ᣬ����
�����ᣬ����![]() ��������ͼ��ʾ��װ���н����绯��
��������ͼ��ʾ��װ���н����绯��
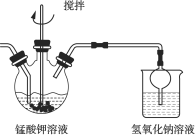
�ٸò��������pH���ƹ��ߣ����ܻᵼ��________��
���ж�����ƿ��![]() ��ȫ��Ӧ��ʵ�鷽���ǣ��ò�����պȡ��Һ������ֽ�ϣ����۲쵽________����ʾ
��ȫ��Ӧ��ʵ�鷽���ǣ��ò�����պȡ��Һ������ֽ�ϣ����۲쵽________����ʾ![]() ����ȫ��Ӧ��
����ȫ��Ӧ��
(3)�����ԭ�������ʵĽǶȷ�������ʵ�����̵��ŵ���________________��
(4)�������ҺΪԭ�ϣ���ȡ![]() �����ʵ�鷽����________________����֪
�����ʵ�鷽����________________����֪![]() ��ˮ��Һ�У�
��ˮ��Һ�У�![]() ���Ͽ�ʼ�ֽ⡣ʵ���б���ʹ�õ��豸����������ˮԡ�ۡ����¸����䣩��
���Ͽ�ʼ�ֽ⡣ʵ���б���ʹ�õ��豸����������ˮԡ�ۡ����¸����䣩��