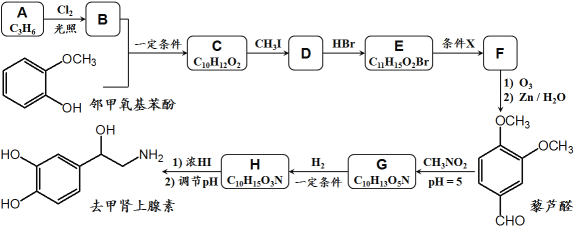
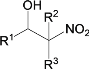
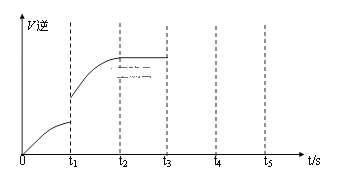
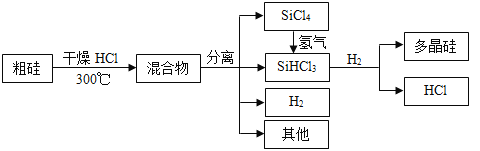
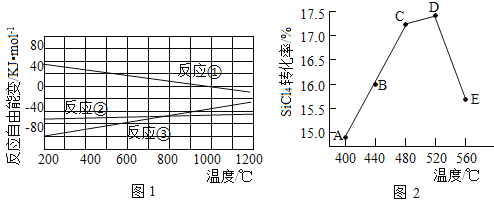
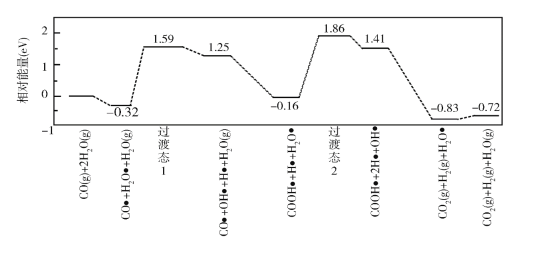
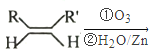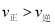��Ŀ����
����Ŀ����֪2CO(g)+O2(g)=2CO2(g) ��H= ��566 kJmol��1
Na2O2(s)+CO2(g) = Na2CO3(s)+1/2O2(g) ��H= ��226 kJmol��1
���б�����ȷ����
A.1molCOȼ��ʱ�ų�������Ϊ283kJmol��1
B.Na2O2(s)+CO(g)=Na2CO3(s)��H=��509kJmol-1
C.Na2O2(s)��CO2(g)��Ӧ�ų�226kJ����ʱ����ת����Ϊ2NA
D.Na2O2��Na2CO3���������Ӹ����Ȳ�ͬ
���𰸡�B
��������
A.1molCOȼ��ʱ�ų�������Ϊ283 kJ����A����
B.���ݸ�˹���ɣ��ڶ�����Ӧ-��һ����Ӧ��![]() �ɵã�Na2O2(s)+CO(g) = Na2CO3(s) ��H= ��509 kJmol-1����B��ȷ��
�ɵã�Na2O2(s)+CO(g) = Na2CO3(s) ��H= ��509 kJmol-1����B��ȷ��
C.Na2O2(s)��CO2(g)��Ӧ�ų�226 kJ����ʱ������0.5mol����������ת����ΪNA����C����
D.Na2O2�к���2�������ӡ�1�����������ӣ�Na2CO3�к���2�������ӡ�1��̼������ӣ������������Ӹ����ȶ���1:2����D����
�ʴ�ѡB��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д�
�����Ŀ