��Ŀ����
��9�֣���һ���ۺ�Fe3O4��ĩ��ɵ����ȼ����ڸ��������������¼���ʹ֮��ַ�Ӧ������Ӧ�����û������ϸ�����ֳɾ��ȵ����ȷݣ���һ���м�����������������Һ����һ���м������������ᣬ��Ӧ��ǰ������m mol NaOH���ų�����0.336 L����������n mol HCl���ų�����V L�����������������ȼ�ֱ��������ϡ�������ã��ɵ�3.36 L���壨��������������ѻ���ɱ�״�������������ȼ���Al������������
28.0%
��1�����ȼ�ֱ����H2SO4����ʱ��������ķ�Ӧ���£�
2Al �� 3 H2SO4 �� Al2(SO4)3 �� 3 H2��
54.0 g 67.2 L
m(Al) 3.36 L
��ʽ�ɽ�ã�m(Al)��2.70 g
���ȷ�Ӧ��Ļ������NaOH�ܷų�H2���������ȷ�ӦAlʣ�࣬��
2Al �� 2NaOH �� 6H2O �� 2Na [Al(HO)4] �� 3 H2��
54.0 g 67.2 L
m(Al) 0.336 L
��ʽ����ã�m��(Al)��0.540 g
���ȼ���Fe3O4����������
3 Fe3O4 �� 8 Al 4 Al2O3 �� 9 Fe
4 Al2O3 �� 9 Fe
696 g 216 g
m(Fe3O4) 2.70g��0.540g
��ʽ����ã�m(Fe3O4)��6.96 g
��w(Al)��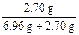 ��100%��28.0%
��100%��28.0%
2Al �� 3 H2SO4 �� Al2(SO4)3 �� 3 H2��
54.0 g 67.2 L
m(Al) 3.36 L
��ʽ�ɽ�ã�m(Al)��2.70 g
���ȷ�Ӧ��Ļ������NaOH�ܷų�H2���������ȷ�ӦAlʣ�࣬��
2Al �� 2NaOH �� 6H2O �� 2Na [Al(HO)4] �� 3 H2��
54.0 g 67.2 L
m(Al) 0.336 L
��ʽ����ã�m��(Al)��0.540 g
���ȼ���Fe3O4����������
3 Fe3O4 �� 8 Al
 4 Al2O3 �� 9 Fe
4 Al2O3 �� 9 Fe696 g 216 g
m(Fe3O4) 2.70g��0.540g
��ʽ����ã�m(Fe3O4)��6.96 g
��w(Al)��
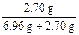 ��100%��28.0%
��100%��28.0%
��ϰ��ϵ�д�
�����Ŀ
