ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥Μ·―ß–Υ»Λ–ΓΉι“ΣΆξ≥…÷–ΚΆ»»ΒΡ≤βΕ®Θ°
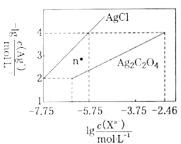
Θ®1Θ© Β―ιΉά…œ±Η”–…’±≠Θ®¥σΓΔ–ΓΝΫΗω…’±≠Θ©ΓΔ≈ίΡ≠ΥήΝœΓΔ≈ίΡ≠ΥήΝœΑεΓΔΫΚΆΖΒΈΙήΓΔΜΖ–Έ≤ΘΝßΫΝΑηΑτΓΔ![]() ―ΈΥαΓΔ
―ΈΥαΓΔ![]() »ή“ΚΘ§…–»±…ΌΒΡ Β―ι≤ΘΝß”ΟΤΖ «____ΓΔ___Θ°
»ή“ΚΘ§…–»±…ΌΒΡ Β―ι≤ΘΝß”ΟΤΖ «____ΓΔ___Θ°
Θ®2Θ© Β―ι÷–ΡήΖώ”ΟΜΖ–ΈΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΫΝΑηΑτΘΩ_____Θ®ΧνΓΑΡήΓ±ΜρΓΑΖώΓ±Θ©Θ§Τδ‘≠“ρ «____Θ°
Θ®3Θ© Β―ι÷–![]() ΒΡ≈®Ε»±»
ΒΡ≈®Ε»±»![]() ΒΡ¥σΘ§Τδ‘≠“ρ «________
ΒΡ¥σΘ§Τδ‘≠“ρ «________
Θ®4Θ©»τ”Ο![]() ¥ζΧφ
¥ζΧφ![]() Θ§Ε‘≤βΕ®ΫαΙϊ________Θ®ΧνΓΑ”–Γ±ΜρΓΑΈόΓ±Θ©”ΑœλΘΜ»τ”Ο¥ΉΥα¥ζΧφ
Θ§Ε‘≤βΕ®ΫαΙϊ________Θ®ΧνΓΑ”–Γ±ΜρΓΑΈόΓ±Θ©”ΑœλΘΜ»τ”Ο¥ΉΥα¥ζΧφ![]() Ήω Β―ιΘ§‘ρ≤βΕ®ΫαΙϊ________Θ®ΧνΓΑΤΪΗΏΓ±ΓΑΤΪΒΆΓ±ΜρΓΑΈό”ΑœλΓ±Θ©Θ°
Ήω Β―ιΘ§‘ρ≤βΕ®ΫαΙϊ________Θ®ΧνΓΑΤΪΗΏΓ±ΓΑΤΪΒΆΓ±ΜρΓΑΈό”ΑœλΓ±Θ©Θ°
Θ®5Θ©ΥϊΟ«Φ«¬ΦΒΡ Β―ι ΐΨί»γœ¬ΘΚ“―÷ΣΘΚ![]() Θ§Ζ¥”ΠΚσ»ή“ΚΒΡ±»»»»ί
Θ§Ζ¥”ΠΚσ»ή“ΚΒΡ±»»»»ί![]() ΈΣ
ΈΣ![]() Θ§ΗςΈο÷ ΒΡΟήΕ»ΨυΈΣ
Θ§ΗςΈο÷ ΒΡΟήΕ»ΨυΈΣ![]() Θ°ΦΤΥψΆξ≥…œ¬±μ_______
Θ°ΦΤΥψΆξ≥…œ¬±μ_______
ΓΨ¥πΑΗΓΩΝΩΆ≤ Έ¬Ε»ΦΤ Ζώ Ϋπ τ“ΉΒΦ»»Θ§»»ΝΩ…Δ ßΒΦ÷¬Έσ≤ν¥σ »Ζ±Θ―ΈΥαΆξ»ΪΖ¥”ΠΘ§ ΙΖ¥”ΠΗϋ≥δΖ÷ Έό ΤΪΒΆ ![]() Θ®Μρ
Θ®Μρ![]() Θ©
Θ©![]()
ΓΨΫβΈωΓΩ
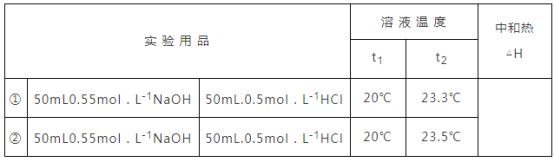
(1)ΗυΨί÷–ΚΆ»»≤βΕ®ΒΡ Β―ι≤Ϋ÷η―Ôϖη“ΣΒΡ“«ΤςΘ§»ΜΚσ≈–ΕœΜΙ»±…ΌΒΡ“«ΤςΘΜ
(2)Ϋπ τΒΦ»»ΩλΘ§»»ΝΩΥπ ßΕύΘΜ
(3)NaOHΒΡ≈®Ε»±»HClΒΡ¥σΘ§ΡΩΒΡ «NaOHΙΐΝΩΘ§»Ζ±Θ»Ζ±Θ―ΈΥαΆξ»ΪΖ¥”ΠΘΜ
(4)ΗυΨίKOH“≤ ««ΩΦνΘ§ΖϊΚœ÷–ΚΆ»»ΒΡΗ≈ΡνΘΜΗυΨί»θΒγΫβ÷ ΒΡΒγάκΈϋ»»ά¥Ζ÷ΈωΘΜ
(5)œ»≈–ΕœΈ¬Ε»≤νΒΡ”––ß–‘Θ§»ΜΚσ«σ≥ωΈ¬Ε»≤νΤΫΨυ÷ΒΘ§‘ΌΗυΨίQ=mcΓςTΦΤΥψΖ¥”ΠΖ≈≥ωΒΡ»»ΝΩΘ§»ΜΚσΗυΨίΓςH=-![]() kJ/molΦΤΥψ≥ωΖ¥”Π»»ΓΘ
kJ/molΦΤΥψ≥ωΖ¥”Π»»ΓΘ
(1)÷–ΚΆ»»ΒΡ≤βΕ®Ιΐ≥Χ÷–Θ§–η“Σ”ΟΝΩΆ≤ΝΩ»ΓΥα»ή“ΚΓΔΦν»ή“ΚΒΡΧεΜΐΘ§–η“Σ Ι”ΟΈ¬Ε»ΦΤ≤βΝΩΈ¬Ε»Θ§Υυ“‘ΜΙ»±…ΌΈ¬Ε»ΦΤΚΆΝΩΆ≤ΘΜ
(2)≤ΜΡή”ΟΜΖ–ΈΆ≠ΥΩΫΝΑηΑτ¥ζΧφΜΖ–Έ≤ΘΝßΫΝΑηΑτΘ§“ρΈΣΆ≠ΥΩΫΝΑηΑτ «»»ΒΡΝΦΒΦΧεΘ§»»ΝΩΥπ ߥσΘΜ
(3)NaOHΒΡ≈®Ε»±»HClΒΡ¥σΘ§ΡΩΒΡ «NaOHΙΐΝΩΘ§»Ζ±Θ»Ζ±Θ―ΈΥαΆξ»ΪΖ¥”ΠΘ§ ΙΖ¥”ΠΗϋ≥δΖ÷ΘΜ
(4)”ΟKOH¥ζΧφNaOHΘ§KOH“≤ ««ΩΦνΘ§ΖϊΚœ÷–ΚΆ»»ΒΡΗ≈ΡνΘ§Ε‘≤βΕ®ΫαΙϊΈό”ΑœλΘΜΕχ”Ο¥ΉΥα¥ζΧφHClΘ§”…”Ύ¥ΉΥαΈΣ»θΥαΘ§Βγάκ–ηΈϋ»»Θ§Έ¬Ε»ΤΪΒΆΘ§‘ρ≤βΕ®ΫαΙϊΤΪΒΆΘΜ
(5)2¥ΈΈ¬Ε»≤νΖ÷±πΈΣΘΚ3.3ΓφΘ§3.5ΓφΘ§2Ήι ΐΨίΕΦ”––ßΘ§Έ¬Ε»≤νΤΫΨυ÷Β=3.4ΓφΘ§50mL0.55molL-1NaOHΚΆ50mL.0.5molL-1HCl÷ ΝΩΚΆΈΣm=100mLΓΝ1g/mL=100gΘ§c=4.18J/(gΓφ)Θ§ΓςT=3.4ΓφΘ§¥ζ»κΙΪ ΫQ=cmΓςTΒΟ…ζ≥…0.025molΒΡΥ°Ζ≈≥ω»»ΝΩQ=4.18J/(gΓφ)ΓΝ100gΓΝ3.4Γφ=1421.2J=1.4212kJΘ§Φ¥…ζ≥…0.025molΒΡΥ°Ζ≈≥ω»»ΝΩ1.4212kJΘ§Υυ“‘…ζ≥…1molΒΡΥ°Ζ≈≥ω»»ΝΩΈΣ![]() =56.8kJΘ§Φ¥ΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»»ΓςH=-56.8kJ/molΓΘ
=56.8kJΘ§Φ¥ΗΟ Β―ι≤βΒΟΒΡ÷–ΚΆ»»ΓςH=-56.8kJ/molΓΘ

 ≥ε¥Χ100Ζ÷ΒΞ‘Σ”≈Μ·ΝΖΩΦΨμœΒΝ–¥πΑΗ
≥ε¥Χ100Ζ÷ΒΞ‘Σ”≈Μ·ΝΖΩΦΨμœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ¥”ΡήΝΩΒΡ±δΜ·ΚΆΖ¥”ΠΒΡΩλ¬ΐΒ»Ϋ«Ε»―–ΨΩΖ¥”ΠΘΚ![]() Θ°
Θ°
Θ®1Θ©ΈΣΝΥΦ”Ωλ’ΐΖ¥”ΠΥΌ¬ Θ§Ω…“‘≤…»ΓΒΡ¥κ ©”–________Θ®Χν–ρΚ≈Θ§œ¬Ά§Θ©Θ°
AΘ° Ι”Ο¥ΏΜ·ΦΝ BΘ° Β±ΧαΗΏ―θΤχΒΡ≈®Ε»
CΘ° Β±ΧαΗΏΖ¥”ΠΒΡΈ¬Ε» DΘ° Β±ΫΒΒΆΖ¥”ΠΒΡΈ¬Ε»
Θ®2Θ©“―÷ΣΗΟΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§œ¬ΆΦΡή’ΐ»Ζ±μ ΨΗΟΖ¥”Π÷–ΡήΝΩ±δΜ·ΒΡ «________Θ°
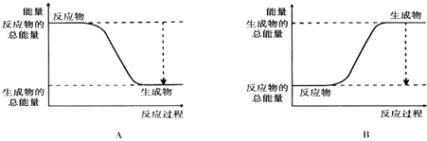
Θ®3Θ©¥”ΕœΦϋΚΆ≥…ΦϋΒΡΫ«Ε»Ζ÷Έω…œ ωΖ¥”Π÷–ΡήΝΩΒΡ±δΜ·Θ°
Μ·―ßΦϋ |
|
|
|
ΦϋΡή |
|
|
|
«κΧν–¥œ¬±μΘΚ
Μ·―ßΦϋ | ΧνΓΑΈϋ ’Γ±ΜρΓΑΖ≈≥ωΓ±ΡήΝΩ | ΡήΝΩ±δΜ· | |
≤πΩΣΜ·―ßΦϋ |
| ΔΌ________ | Δή________ |
| |||
–Έ≥…Μ·―ßΦϋ |
| ΔΎ________ | Δί________ |
ΉήΡήΝΩ±δΜ· | Δέ________ | Δό________ | |
ΓΨΧβΡΩΓΩœ÷”–Ρ≥–©ΕΧ÷ήΤΎ‘ΣΥΊΒΡ–‘÷ Μρ‘≠Ή”ΫαΙΙ–≈œΔ»γ±μΥυ ΨΓΘ
‘ΣΥΊ | ‘ΣΥΊ–‘÷ Μρ‘≠Ή”ΫαΙΙ–≈œΔ |
T | M≤ψ…œ”–6ΗωΒγΉ” |
X | ΉνΆβ≤ψΒγΉ” ΐ «¥ΈΆβ≤ψΒγΉ” ΐΒΡ2±Ε |
Y | ≥ΘΈ¬œ¬ΒΡΒΞ÷ ΈΣΥΪ‘≠Ή”Ζ÷Ή”Θ§ΤδΦρΒΞ«βΜ·ΈοΒΡΥ°»ή“Κ≥ Φν–‘ |
Z | ‘ΣΥΊΒΡΉνΗΏ’ΐΦέ «+7 |
(1)‘ΣΥΊXΒΡ“Μ÷÷Ά§ΈΜΥΊΩ…≤βΕ®ΈΡΈοΡξ¥ζΘ§’β÷÷Ά§ΈΜΥΊΒΡΖϊΚ≈ «______ΓΘ
(2)‘ΣΥΊY”κ«β‘ΣΥΊΡή–Έ≥…ΝΫ÷÷≥ΘΦϊΒΡ10ΒγΉ”ΈΔΝΘΘ§«“‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§Τδ÷–“Μ÷÷ΈΔΝΘ”κOHΖ¥”ΠΩ…“‘ΉΣΜ·ΈΣΝμ“Μ÷÷ΈΔΝΘΘ§ΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ_________________ΓΘ
(3)‘ΣΥΊZ”κ‘ΣΥΊTœύ±»Θ§Ζ«Ϋπ τ–‘Ϋœ«ΩΒΡ «______(Χν‘ΣΥΊΖϊΚ≈)Θ§œ¬Ν–±μ ωΡή÷ΛΟς’β“Μ ¬ ΒΒΡ «_______(Χν–ρΚ≈)ΓΘ
aΘ°≥ΘΈ¬œ¬ZΒΡΒΞ÷ ΚΆTΒΡΒΞ÷ Ή¥Χ§≤ΜΆ§
bΘ°ZΒΡΦρΒΞ«βΜ·Έο±»TΒΡΦρΒΞ«βΜ·ΈοΈ»Ε®
cΘ°“ΜΕ®ΧθΦΰœ¬ZΚΆTΒΡΒΞ÷ ΕΦΡή”κ«β―θΜ·ΡΤ»ή“ΚΖ¥”Π
(4)TΓΔXΓΔYΓΔZΥΡ÷÷‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·Έο÷–Μ·―ß–‘÷ Οςœ‘≤ΜΆ§”ΎΤδΥϊ»ΐ÷÷ΒΡ «____(ΧνΕ‘”ΠΥαΒΡΜ·―ß Ϋ)Θ§άμ”… «__________ΓΘ