��Ŀ����
(1)�⻯�����ȼ�ԣ��ڶ�����__________��__________H2O��HF��
��������SiH4��PH3��__________��__________��
(2)������Ķ��ԣ�PH3��NH3 H2S_______H2O��CS2__________CO2 CCl4��CF4(ѡ���)��
���ǿ�ѧ���ǿ�ʼ��ע���������ں�F��Cl�Ļ������ϡ�
(3)��֪CCl4�ķе�Ϊ76.8�档CF4�ķе�Ϊ��128 �档��������ķе㷶Χ������䡣�����ϳ�ʱ�䷴�����飬һ���µ���������ﰺCF2Cl2���ڵ����ˣ��������ƵĻ�������___________��
(4)Ȼ�����������������˵����ijһ����������___________�������������ڱ���Ԫ�ؼ��仯�����___________�仯����������������Ŀ�ѧ˼ά������ֵ�ý���ġ�
�ٶ��� �ڷе� ����ȼ�� ��ˮ���� ����ɫ
a.�٢ڢ� b.�ڢܢ� c.�ڢۢ�
(1)CH4��NH3��H2S��HCl (2)�� �� (3)CFCl3(��CF3Cl) (4)ʹ������������ֿն� a
����������������ʹ���Լ��Ի�������Ⱦ�Ϳ������������˼·�������е���Ŀһ�£�������Ҫ�����֪ʶ�Ƿdz��ġ�(1)��������ں�������Ԫ�ص��⻯�ﲻ������ո�(2)�������Ϣ����PH3��NH3��ͬ����Ԫ�����γɽṹ��������Ƶ����ʣ����϶��¶�������(3)��CF2Cl2����ɿ�������������Ƶ����ʣ���CF3Cl��CFCl3��(4)���ﰺCF2Cl2����������Ի�����Σ�����������ƻ������㣬���Կ����������Ҫע���������ʵĶ��ԡ��е㡢��ȼ�ԡ��Լ��Ի�����Σ���Ե����ء�

��ϰ��ϵ�д�
�����Ŀ
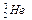 ��ÿ�ٶ�
��ÿ�ٶ�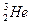 �˾۱����ͷų��������൱��Ŀǰ����һ�����ĵ��������ڵ����ϣ���Ԫ����Ҫ��
�˾۱����ͷų��������൱��Ŀǰ����һ�����ĵ��������ڵ����ϣ���Ԫ����Ҫ��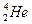 ����ʽ���ڡ�����˵����ȷ����
����ʽ���ڡ�����˵����ȷ����
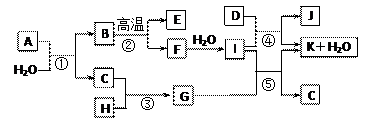
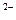 ��C
��C �����Ӱ뾶A2һ����C
�����Ӱ뾶A2һ����C