��Ŀ����
A��B��C��D ���ֶ�����Ԫ�أ�ԭ�������������� A ԭ�ӵ���������� 5 �����ӣ� B �������Ӻ� C �������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ��� E , D ��L ����������� K��M �������Ӳ��ϵĵ�����֮�͡� ( 1 ) A ������������ˮ������___________���ѧʽ��;
( 2 ������ E ���������Ļ�ѧ����______________��д�� C��D ��Ԫ���γɵĻ����� C2D �ĵ���ʽ______________________��
( 3 ����ʢ�� 48mL AB��AB2������������������ˮ�У�ͬ�¡�ͬѹ�£�����Һ���ȶ������������������Ϊ 24 mL����ԭ��������� AB ���������Ϊ__________��
��ÿ�� 2 �֣��� 8 �֣�
( 2 ������ E ���������Ļ�ѧ����______________��д�� C��D ��Ԫ���γɵĻ����� C2D �ĵ���ʽ______________________��
( 3 ����ʢ�� 48mL AB��AB2������������������ˮ�У�ͬ�¡�ͬѹ�£�����Һ���ȶ������������������Ϊ 24 mL����ԭ��������� AB ���������Ϊ__________��
��ÿ�� 2 �֣��� 8 �֣�
( l ) HNO3 , ( 2 �����Ӽ������Ǽ��ԣ����ۼ���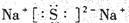 ��
��
( 3 ) 25 %
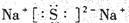 ��
��( 3 ) 25 %
A ��ԭ��������С�� A ԭ�ӵ���������� 5 �����ӣ�˵���� N ; E �ǵ���ɫ�Ĺ��� Na2O2���� B ��O�� AB �� AB2�ֱ��� NO �� NO2; D �� L ����������� K��M �������Ӳ��ϵĵ�����֮���� S ���� NO ���Ϊx mL , NO2���Ϊy mL �����з���ʽ��x + y�� 48 , x + y =" 24" �����x�� 12 ��

��ϰ��ϵ�д�
�����Ŀ