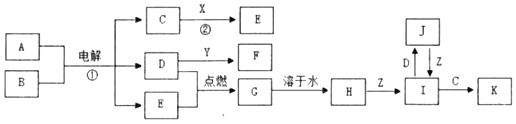
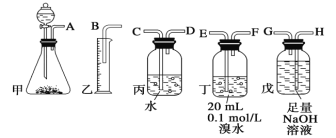
��Ŀ����
����Ŀ������Ԫ�صĻ��ϼ��Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ�ͼ����Ԫ�صij������ϼ��벿���������Ķ�Ӧ��ϵ��
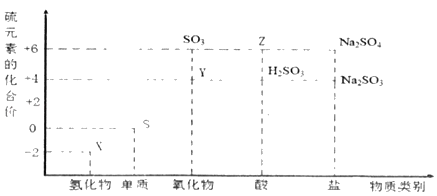
(1)����Ԫ�ػ��ϼ۱仯�ĽǶȷ�����ͼ�м������������л�ԭ�Ե�������Ϊ_____(�ѧʽ)��
(2)��X��Y��Ͽ����ɵ���ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽΪ_____��
(3)Z��Ũ��Һ��̼��һ�������¿��Է�����Ӧ��������Z��_____�ԡ�
(4)д������Ũ�����ڼ��������·�Ӧ�Ļ�ѧ����ʽ_____��
(5)Na2S2O3����Ҫ�Ļ���ԭ�ϡ���������ԭ��Ӧ�ĽǶȷ����������Ʊ�Na2S2O3�ķ��������Ͽ��е���_____(����ĸ)��
a��Na2SO3+S b��Na2S+S c��SO2+Na2SO4 d��Na2SO3+Na2SO4
���𰸡�SO2 2H2S+SO2��3S��+2H2O ǿ���� S+2H2SO4(Ũ)![]() 3SO2��+2H2O a
3SO2��+2H2O a
��������
��1��Ԫ�ش����м��̬���Ⱦ������������л�ԭ�ԣ�
��2��XΪH2S��YΪSO2������2H2S+SO2=3S��+2H2O��
��3��ZΪŨ���ᣬC��Ũ���ᷴӦ���ɶ�����̼�����������ˮ��
��4������Ũ�����ڼ��������·�Ӧ���ɶ��������ˮ��
��5���Ʊ�Na2S2O3ʱ����Ӧ����SԪ�صĻ��ϼ۴���+2��С��+2��
(1)�����м��̬��Ԫ�ؾ��������Ժͻ�ԭ�ԣ���ͼ�м������������л�ԭ�ԵĻ�������SO2��H2SO3��Na2SO3��������ΪSO2��
�ʴ�Ϊ��SO2��
(2)XΪH2S��YΪSO2��XΪ��ԭ����YΪ�����������������뻹ԭ�������ʵ���֮��Ϊ1��2��������Ӧ�Ļ�ѧ����ʽ��2H2S+SO2��3S��+2H2O��
�ʴ�Ϊ��2H2S+SO2��3S��+2H2O��
(3)ZΪŨ���ᣬC��Ũ���ᷴӦ���ɶ�����̼�����������ˮ��������Ũ�����ǿ�����ԣ�
�ʴ�Ϊ��ǿ������
(4)����Ũ�����ڼ��������·�Ӧ���ɶ��������ˮ����Ӧ�Ļ�ѧ����ʽ��S+2H2SO4(Ũ)![]() 3SO2��+2H2O��
3SO2��+2H2O��
�ʴ�Ϊ��S+2H2SO4(Ũ)![]() 3SO2��+2H2O��
3SO2��+2H2O��
(5)�Ʊ�Na2S2O3ʱ����Ӧ����SԪ�صĻ��ϼ۴���+2��С��+2��ֻ��a���ϣ�
�ʴ�Ϊ��a��