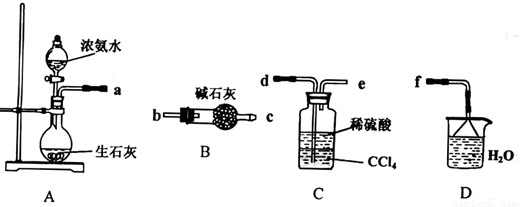
��Ŀ����
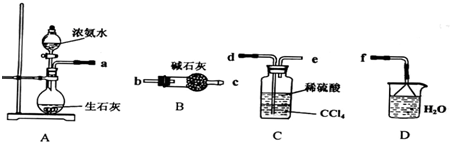
��12�֣��������ƿ������ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
��֪CaO2��8H2O�ʰ�ɫ������ˮ��������350�����ҿ�ʼ�ֽ�ų�������
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����� ��
��3������ʱ���ñ�ˮ�����¶���0�����ң������ԭ���ǣ�д�����֣���
�� ���� ��
��4���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol��L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
[��֪��I2+2S2O32��= 2I��+S4O62��]
��CaO2����������Ϊ (����ĸ��ʾ)��
��ijͬѧ��һ���͵ڶ����IJ������ܹ淶������������̫����������õ�CaO2�������������� �������Ӱ�족����ƫ�͡���ƫ�ߡ�����ԭ����_______________��
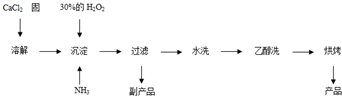
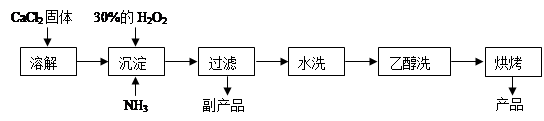
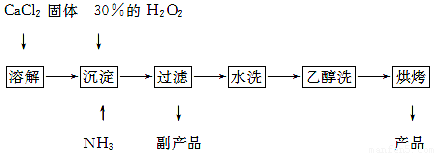
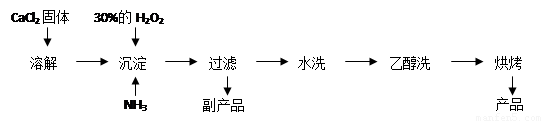
��1��CaCl2+H2O2+2NH3+8H2O = CaO2��8H2O��+2NH4Cl ��2�֣�
��2����ȡ���һ��ϴ��Һ�������Թ��У��ٵμ�ϡ�����ữ����������Һ�����Ƿ������ɫ��������2�֣�
��3�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ�1�֣���
�ڸ÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��8H2O���ʣ�1�֣���
��4����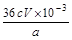 ����2�֣�
����2�֣�
��ƫ��(2��)�������������¿����е�O2Ҳ����KI����ΪI2��ʹ���ĵ�Na2S2O3���࣬�Ӷ�ʹ��õ�CaO2����������ƫ�ߡ�(2��)
����������1�����ݷ�Ӧ���а�����֪��������Ӧ�û����Ȼ�泥����Է���ʽΪCaCl2+H2O2+2NH3+8H2O = CaO2��8H2O��+2NH4Cl��
��2��ˮϴ��Ŀ���dz�ȥ��������������ӣ����Կ���ͨ�����������ӵķ���������֤������ȡ���һ��ϴ��Һ�������Թ��У��ٵμ�ϡ�����ữ����������Һ�����Ƿ������ɫ������
��3������˫��ˮ�����ֽ⣬�����¶ȵͿɼ��ٹ�������ķֽ⣬��߹�������������ʣ���һ����÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2��8H2O���ʡ�
��4����CaO2���������ԣ��ܱ��⻯���������ɵ��ʵ⣬����ʽΪCaO2��2KI��2H2SO4=CaSO4��K2SO4��I2��2H2O�����Ը��ݷ���ʽ��֪CaO2��2Na2S2O3�����CaO2�����ʵ�����0.0005cVmol������CaO2����������Ϊ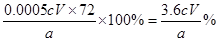 ��
��
��������ٹ������������е�����������������Ҳ����KI����ΪI2��ʹ���ĵ�Na2S2O3���࣬�Ӷ�ʹ��õ�CaO2����������ƫ�ߡ�

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�