��Ŀ����
18��A��B��C��D��E��F��G��H�����л����ת����ϵ��ͼ��ʾ����������A�ڱ�״�����ܶ�Ϊ1.25g/L����̼Ԫ�ص���������Ϊ85.7%��E�ձ������ʳ���У��粤�˵ȣ���F�����к���һ����Ԫ��������H�н���C��H��O����Ԫ�أ���ԭ�Ӹ�����Ϊ1��3��3��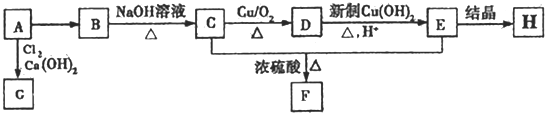
�ش��������⣺
��1��C�Ľṹ��ʽΪHOCH2CH2OH������H�Ļ�ѧʽΪH2C2O4.2H2O��
��2������ת�������漰���л���ѧ��Ӧ�У�����ȡ����Ӧ���Ǣڢݣ�����ţ���
��A��B ��B��C ��C��D ��D��E ��C��E��F
��3��д��D������Cu��OH��2����Һ��Ӧ�ķ���ʽ��OHC-CHO+4Cu��OH��2+2NaOH$\stackrel{��}{��}$NaOOC-COONa+2Cu2O��+6H2O��д��C��E���ɸ߷��ӻ�����Ļ�ѧ����ʽ��
 ��
����4������ȷ���л���B�й����������ʵ�鷽����ȡ����B����NaOH��Һ���ȣ��ټ������ữ�����AgNO3��Һ���۲����ɳ�����ɫ���۲����ɳ�����ɫ���ȷ����
��5���л���W��F������ͬ�Ĺ����ţ��������༰��Ŀ����������ɱ�F���ĸ�̼ԭ�ӣ�Ҳ����һ����Ԫ������1H-NMR��ֻ��һ���壬д����������������W�Ľṹ��ʽ��
 ��
����6��G����Է�������Ϊ44����A��G��Ӧ�����У��������Ʊ�G��ԭ��������Ϊ��25.4%��
���� ����A�ڱ�״�����ܶ�Ϊ1.25g/L����Ħ������=1.25g��22.4g/mol=28g/mol����̼Ԫ�ص���������Ϊ85.7%��A��̼ԭ�Ӹ���=$\frac{28��85.7%}{12}$=2����A���л������A��CH2=CH2��
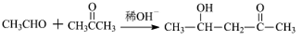
B��NaOH��ˮ��Һ��Ӧ����C��C�ܱ�������������D��D������������ͭ��������E���ᣬ��D�к���ȩ����C�к��д��ǻ���C��E����������Ӧ����F��F�����к���һ����Ԫ��������̼ԭ���غ�֪��F��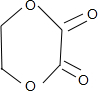 ������C��HOCH2CH2OH��E��HOOCCOOH��D��OHCCHO��B����������ˮ��Һ����ȡ����Ӧ����HOCH2CH2OH����B��±������A���������巢���ӳɷ�Ӧ����B��
������C��HOCH2CH2OH��E��HOOCCOOH��D��OHCCHO��B����������ˮ��Һ����ȡ����Ӧ����HOCH2CH2OH����B��±������A���������巢���ӳɷ�Ӧ����B��
����H�н���C��H��O����Ԫ�أ���ԭ�Ӹ�����Ϊ1��3��3��E��HOOCCOOH�������ʽΪ��C2H2O4��H���Ҷ���Ľᾧˮ�����C��H��Oԭ�Ӹ�����Ϊ1��3��3������̼ԭ���غ�֪���ýᾧˮ�����к���2��̼ԭ�ӣ���H������6��O������6�����Ըýᾧˮ�����к�2��H2O��
G����Է�������Ϊ44��A���������������Ʒ�Ӧ����G��GΪCH3CHO���ݴ˷������
��� �⣺����A�ڱ�״�����ܶ�Ϊ1.25g/L����Ħ������=1.25g��22.4g/mol=28g/mol����̼Ԫ�ص���������Ϊ85.7%��A��̼ԭ�Ӹ���=$\frac{28��85.7%}{12}$=2����A���л������A��CH2=CH2��
B��NaOH��ˮ��Һ��Ӧ����C��C�ܱ�������������D��D������������ͭ��������E���ᣬ��D�к���ȩ����C�к��д��ǻ���C��E����������Ӧ����F��F�����к���һ����Ԫ��������̼ԭ���غ�֪��F�� ������C��HOCH2CH2OH��E��HOOCCOOH��D��OHCCHO��B����������ˮ��Һ����ȡ����Ӧ����HOCH2CH2OH����B��±������A���������巢���ӳɷ�Ӧ����B��
������C��HOCH2CH2OH��E��HOOCCOOH��D��OHCCHO��B����������ˮ��Һ����ȡ����Ӧ����HOCH2CH2OH����B��±������A���������巢���ӳɷ�Ӧ����B��
����H�н���C��H��O����Ԫ�أ���ԭ�Ӹ�����Ϊ1��3��3��E��HOOCCOOH�������ʽΪ��C2H2O4��H���Ҷ���Ľᾧˮ�����C��H��Oԭ�Ӹ�����Ϊ1��3��3������̼ԭ���غ�֪���ýᾧˮ�����к���2��̼ԭ�ӣ���H������6��O������6�����Ըýᾧˮ�����к�2��H2O��
G����Է�������Ϊ44��A���������������Ʒ�Ӧ����G��GΪCH3CH2OH��
��1��C�Ľṹ��ʽΪHOCH2CH2OH������H�Ļ�ѧʽΪH2C2O4.2H2O��
�ʴ�Ϊ��HOCH2CH2OH��H2C2O4.2H2O��
��2���������Ϸ���֪������ȡ����Ӧ����B��C��C��E��F��
��ѡ���ڢݣ�
��3���Ҷ�ȩ������������ͭ��Ӧ�����Ҷ����ơ�������ͭ��ˮ����Ӧ����ʽΪ��
OHC-CHO+4Cu��OH��2+2NaOH$\stackrel{��}{��}$NaOOC-COONa+2Cu2O��+6H2O��
��Ũ���������������������£��Ҷ�����Ҷ�������������Ӧ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ��OHC-CHO+4Cu��OH��2+2NaOH$\stackrel{��}{��}$NaOOC-COONa+2Cu2O��+6H2O�� ��
��
��4��B�к���±ԭ�ӣ�±ԭ�Ӻ���������ˮ��Һ����ȡ����Ӧ���ɴ�����±�ᣬ��±��������ữ����������Һ�������ֽⷴӦ���ɳ��������ݳ�����ɫ�ж�±ԭ�ӣ���������鷽���ǣ�ȡ����B����NaOH��Һ���ȣ��ټ������ữ�����AgNO3��Һ���۲����ɳ�����ɫ��
�ʴ�Ϊ��ȡ����B����NaOH��Һ���ȣ��ټ������ữ�����AgNO3��Һ���۲����ɳ�����ɫ��
��5��F���Ҷ����Ҷ������л���W��F��ͬϵ�������ɱ�F��4��̼ԭ�ӣ�Ҳ������Ԫ������1H-NMR��ͼ��ֻ��һ����˵��ֻ��һ�����͵���ԭ�ӣ����Է�������������W�Ľṹ��ʽΪ��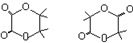 ���ʴ�Ϊ��
���ʴ�Ϊ��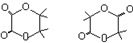 ��
��
��6��G��CH3CHO��A����ϩ�������ķ�ӦΪ
2CH2=CH2+2Cl2+2Ca��OH��2$\stackrel{һ��������}{��}$2CH2CHO+CaCl2+Ca��ClO��2+2H2O��
ԭ��������=$\frac{44��2}{28��2+71��2+74��2}��100%$=25.4%��
�ʴ�Ϊ��25.4%��
���� ���⿼���л����ƶϣ����ؿ���ѧ�������жϡ��������������ݷ�Ӧ������������ɡ��������ʽ����ƶϣ��ѵ��ǣ�6����ԭ�������ʵļ��㣬ע��±������±ԭ�Ӽ��鷽�������������������¼��飬��Ŀ�Ѷ��еȣ�

 �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�| A�� | BaSO4��BaCO3��������ˮ���������������͡� | |
| B�� | �ִ�����Ǻ�������������Ҫ�����˵绯ѧ��ʴ | |
| C�� | ��ʯ��ˮ������������֪�ı�ʯ�����仯ѧ�ɷֲ�ͬ | |
| D�� | �������������������ھ�ˮ |
| �� �� | ��A��C6H5OH ��B��CH3COOCH3 ��C��CH3=CHCOOH ��D��CH3CH2Br |
| �� �� | ����ˮ ��FeCl3��Һ ��NaOH��Һ ��HCHO��Һ |
��2��������A�������Тڿ��Է�����ɫ��Ӧ���˷�Ӧ�������������ʵ�����飮
��3��������A�����������������ʷ�����Ӧ��
��4�������Т����������A����ȡ����Ӧ����C�����ӳɷ�Ӧ��
��5�������е�һ�����ʺ������е�һ�����ʿ��Է������۷�Ӧ����һ�ַۺ�ɫ���߷��ӻ�����÷�Ӧ�ķ���ʽΪ
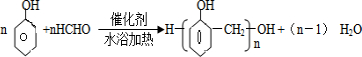 ��
�� | A�� | �����Բⶨijδ֪Ũ�ȵĴ�����Һ�д���ĵ��볣��Ka��Ӧ����ʵ���������Լ�������ֽ��Ϊ���к͵ζ�ʵ�顢pH��ֽ | |
| B�� | ��0.1mol/L��NaOH��Һ��0.5mol/L��CuSO4��Һ���������Ƶ�������ͭ����Һ�����ڼ�����ѿ���ǻ�ԭ���� | |
| C�� | ���ܱ������м���1.5mol H2��0.5mol N2��ַ�Ӧ�ɵõ�NH3������ΪNA | |
| D�� | ��״���£�33.6L���ȼ����к�����ԭ�ӵ���ĿΪ3NA |
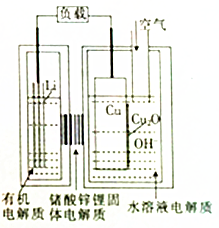 ������AIST���������о�һ�֡����������ͳɱ����-ͭ����ȼ�ϵ�أ��õ��ͨ��һ�ָ��ӵ�ͭ��ʴ�����������������зŵ����Ϊ2Li+Cu2O+H2O=2Cu+2Li++2OH-������˵������ȷ���ǣ�������
������AIST���������о�һ�֡����������ͳɱ����-ͭ����ȼ�ϵ�أ��õ��ͨ��һ�ָ��ӵ�ͭ��ʴ�����������������зŵ����Ϊ2Li+Cu2O+H2O=2Cu+2Li++2OH-������˵������ȷ���ǣ�������| A�� | �ŵ�ʱ��Li+������������Cu���ƶ� | |
| B�� | �ŵ�ʱ�������ĵ缫��ӦʽΪCu2O+H2O+2e-=Cu+2OH- | |
| C�� | ͨ����ʱ��ͭ����ʴ���������Cu2O | |
| D�� | ������Ӧ�����У�ͭ�൱�ڴ��� |
| A�� | �ⶨ�е� | B�� | �⾲���Һ��Ӱ�� | ||
| C�� | �ⶨ�����ܶ� | D�� | ���״��������Ħ����� |
| A�� | H2S+2NO3-+2H+=2NO2��+S��+2H2O | |
| B�� | 3 H2S+2NO3-+2H+=2NO��+3S��+4H2O | |
| C�� | 3Fe3++3NO3-+6H2S=3NO��+6S��+3Fe2++6H2O | |
| D�� | Fe3++3NO3-+5H2S+2H+=3NO��+5S��+Fe2++6H2O |
| A�� | 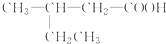 2-�һ����� 2-�һ����� | B�� | CH3-CH2-CH2-CH2OH 1-���� | ||
| C�� | 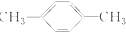 ����ױ� ����ױ� | D�� | 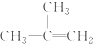 2-��-2-��ϩ 2-��-2-��ϩ |

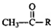 $\stackrel{ϡOH-}{��}$
$\stackrel{ϡOH-}{��}$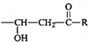
 ��G��H�ķ�Ӧ����Ϊ������Ӧ
��G��H�ķ�Ӧ����Ϊ������Ӧ ����F��������Cu��OH��2����Һ�����ȣ��۲쵽�������dz��ֺ�ɫ����
����F��������Cu��OH��2����Һ�����ȣ��۲쵽�������dz��ֺ�ɫ����