��Ŀ����
̼��̼�Ļ�����㷺�Ĵ��������ǵ������С�
��1���������з�Ӧ�������仯ʾ��ͼ��2C(s) +O2(g) =2CO(g) ��H= ��
��2�������Ϊ2L���ܱ������У�����1 mol CO2��3mol H��һ�������·�����Ӧ�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H<O���CO2(g)��CH3OH(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��
CH3OH(g)+H2O(g) ��H<O���CO2(g)��CH3OH(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��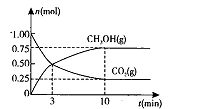
�ٴӷ�Ӧ��ʼ��ƽ�⣬H2O��ƽ����Ӧ����v(H2O)= ��
�����д�ʩ����ʹ��ѧƽ��������Ӧ�����ƶ����� �����ţ���
A�������¶� B����CH3OH��g����ʱҺ���Ƴ�
C��ѡ���Ч���� D���ٳ���l mol CO2��4 mol H2
��3��CO2����ˮ����̼�ᡣ��֪�������ݣ�
| ������� | H2CO3 | NH3��H2O |
| ����ƽ�ⳣ���� 25�棩 | Ka1 = 4��30 �� 10һ7 Ka2= 5��61�� 10һ11 | Kb = 1��77�� 10һ5 |
���г�����1 mol��L��1��( NH4)2CO3��Һ����֪��
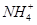 ˮ���ƽ�ⳣ��Kh=Kw/Kb��
ˮ���ƽ�ⳣ��Kh=Kw/Kb��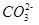
��һ��ˮ���ƽ�ⳣ��Kh=Kw/Ka2��
���жϸ���Һ�� ����ᡱ�����С��� ������ԣ�д������Һ��
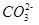 ������һ��ˮ������ӷ���ʽ ��
������һ��ˮ������ӷ���ʽ ������������֮��Ĺ�ϵʽ��������ȷ���� ��
A��
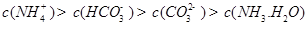
B��

C��
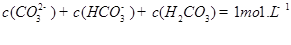
D��
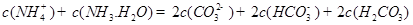
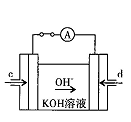
��4���ݱ�������ѧ����ʵ���������Ƴ���ȼ�ϵ�صķ�Ӧ�����У���������缫������CO��O2Ϊԭ�����ɵ�ء�ԭ����ͼ��ʾ��ͨ��CO�Ĺܿ��� ���c����d������д���õ缫�ĵ缫��Ӧʽ�� ��
��1��-221.0kJ/mol (2)��0.0375mol/(L��min) �� B D  (3) �ټ� CO32-+H2O
(3) �ټ� CO32-+H2O HCO3-+OH- �� C D (4) d CO-2e-+4OH-=CO32-+2H2O
HCO3-+OH- �� C D (4) d CO-2e-+4OH-=CO32-+2H2O
�������������������һ���ۺ��Խ�ǿ������ѧ������ѧ֪ʶ���ۺ�Ӧ����������1�����������仯��ͼ��ȷ��C(s)+O2(g)=CO2(g) ��H="-393.5kJ/mol," C(s)+CO2(g)="2CO" (g) ��H=+172.5kJ/mol,�ɸ�˹���ɵ�2C(s) +O2(g) =2CO(g) ��H=-221.0kJ/mol����2�����ݻ�ѧ��Ӧ�����ʱ���ʽ����ȷ����Ӧv(H2O)����3�����̼���Ka1 = 4��30 �� 10һ7��NH3��H2O��Kb = 1��77�� 10һ5��֪( NH4)2CO3��Һ��ʾ���ԣ����������غ㣬ѡ��C��D��ȷ����4����ԭ���ԭ������Ϊ�������ҺΪKOH������ȷ���缫��ӦʽΪCO-2e-+4OH-=CO32-+2H2O��
���㣺���黯ѧ��Ӧ�е������仯����ѧ��Ӧ���ʼ���ѧƽ����ƶ��������ˮ�⡢����Ũ�ȵĴ�С�Ƚϡ�

�±��е��������ƻ�1 mol�����еĻ�ѧ�������ĵ�����(kJ):
| ���� | Cl2 | Br2 | I2 | HCl | HBr | HI | H2 |
| ����/kJ | 243 | 193 | 151 | 432 | 366 | 298 | 436 |
�����������ݻش�(1)~(5)�⡣
(1)�������ʱ������е�������͵�������������
A.H2 B.Cl2 C.Br2 D.I2
(2)�����⻯����,���ȶ���������������
A.HCl����������B.HBr����������C.HI
(3)X2+H2
 2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________��
2HX(X����Cl��Br��I )�ķ�Ӧ�����ȷ�Ӧ���Ƿ��ȷ�Ӧ?��:��______________�� (4)��ͬ������,X2(X����Cl��Br��I)�ֱ���������Ӧ,�����ĵ����ʵ���������ʱ,�ų������յ�����������������������
(5)�����ϱ��е�����,������ȷ�ش������(4)��?
��:����������,��ĸ�������______________________��
�о�SO2��CO�ȴ�����Ⱦ��Ĵ��������þ����ش����塣
��.�����Ƽ�ѭ�������ѳ�������SO2���÷���Na2SO3��Һ��Ϊ���ռ������չ���pH��n��SO��?n��HSO3-���仯��ϵ���±���
| n��SO32-��?n��HSO3-�� | 91:9 | 1:1 | 9:91 |
| pH | 8.2 | 7.2 | 6.2 |
��2��������Һ������ʱ����Һ������Ũ�ȹ�ϵ��ȷ���� __��
a��c��Na������2c��SO32-����c��HSO3-��
b��c��Na������c��HSO3-������c��SO32-����c��H������c��OH����
c��c��Na������c��H������c��HSO3-����c��SO32-����c��OH����
��3����ij��Һ�к�3 mol Na2SO3����ε���һ����ϡHCl��ǡ��ʹ��Һ��Cl����HSO3-���ʵ���֮��Ϊ2?1�������������n��HCl��Ϊ __mol��
��.CO�����ںϳɼ״�����Ӧԭ��Ϊ
CO��g����2H2��g��
 CH3OH��g����
CH3OH��g������4�����ݻ�Ϊ2 L���ܱ�������ͨ��0.2 mol CO,0.4 mol H2���ﵽƽ��ʱ��COת����Ϊ50%������¶��µ�ƽ�ⳣ��Ϊ __���ټ���1.0 mol CO�����´ﵽƽ�⣬CO��ת���� __���������䡱��С������ƽ����ϵ��CH3OH��������� __����������䡱��С������
��5����֪CH3OH��g����H2O��g��=CO2��g����3H2��g����
H2��g����
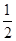 O2��g��=H2O��g������H����241.8 kJ/mol��
O2��g��=H2O��g������H����241.8 kJ/mol���йؼ����������£�����λ��kJ/mol��
| ��ѧ�� | H��H | H��O | C��H | C��O | C=O |
| ���� | 435 | 463 | 413 | 356 | 745 |
д���״�������ȫȼ��������̬ˮ���Ȼ�ѧ����ʽ�� __��
�״�ȼ�Ϸ�Ϊ�״����ͺͼ״����͡���ҵ�Ϻϳɼ״��ķ����ܶࡣ
��1��һ�������·�����Ӧ��
��CO2��g�� +3H2��g�� ��CH3OH��g��+H2O��g�� ��H1
��2CO��g�� +O2��g�� ��2CO2��g�� ��H2
��2H2��g��+O2��g�� ��2H2O��g�� ��H3
��CO��g�� + 2H2��g��  CH3OH��g�����ġ�H�� ��
CH3OH��g�����ġ�H�� ��
��2�����ݻ�Ϊ2L���ܱ������н��з�Ӧ�� CO��g��+2H2��g�� CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����
CH3OH��g�� �������������䣬��300���500��ʱ�����ʵ���n��CH3OH�� �뷴Ӧʱ��t�ı仯������ͼ��ʾ���÷�Ӧ�ġ�H 0 ����>��<��=����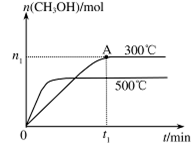
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��____________������ĸ����
| A����������� |
| B�������¶� |
| C�������¶� |
| D��ʹ�ú��ʵĴ��� |
��4��CH4��H2O�ڴ������淢����ӦCH4+H2O
 CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣���
CO+3H2��T��ʱ����1 L�ܱ�������Ͷ��1 mol CH4��1 mol H2O��g����5Сʱ���÷�Ӧ��ϵ�ﵽƽ��״̬����ʱCH4��ת����Ϊ50% ��������¶��µ�ƽ�ⳣ�� ���������С�������λ���֣�����5���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�����������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺
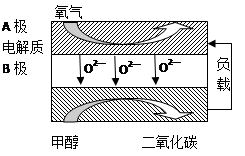
��B���ĵ缫��ӦʽΪ ��
�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������·��ת��1mole- ʱ��ʵ�������ĵļ״��������������ϴ���ԭ���� ��
��6��25��ʱ������Ƶ�Ksp=4.0��10-8,̼��Ƶ�Ksp=2.5��10-9����20ml̼��Ƶı�����Һ����μ���8.0��10-4 mol��L-1�IJ������Һ20ml���ܷ�������� ����ܡ�����
 2CO2(g)+ N2(g) ��H��0��
2CO2(g)+ N2(g) ��H��0��
 N2O4(g) ��H����56.9 kJ/mol ��
N2O4(g) ��H����56.9 kJ/mol �� 2N2(g) + 3H2O(g)��
2N2(g) + 3H2O(g)��
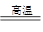 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��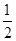 O2(g)=H2O(g)����H����242.0 kJ��mol��1
O2(g)=H2O(g)����H����242.0 kJ��mol��1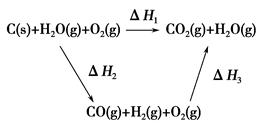
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H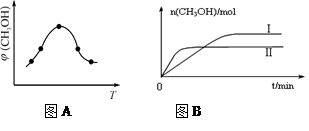
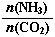 ��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ ��
��3����ƽ��ʱCO2��ת����Ϊ60%����NH3��ƽ��ת����Ϊ �� N2(g)��2CO2(g) ��H=��a kJ��mol-1��
N2(g)��2CO2(g) ��H=��a kJ��mol-1��