��Ŀ����
17����1����Ӧ2KMnO4+16HCl��Ũ���T2MnCl2+2KCl+5Cl2��+8H2O�У�����������KMnO4������������Cl2���ѧʽ������������71gCl2�������ĵ�HCl��3.2mol��
��������HCl��2mol��ת�Ƶ��ӵ����ʵ�����2mol��
��2��ij��ɫ����Һ������Ӧ�ų�H2������Һ�п��ܺ��д�����H+��K+��Mg2+��Cl-��OH-��SO32-��CO32-�����ƶϣ�
��һ���������ɫ����Һ��һ����H+��Cl-��������K+��Mg2+���ӣ�
�ڶ����������ɫ����Һ��һ����OH-��K+��������Cl-��SO32-��CO32-���ӣ�
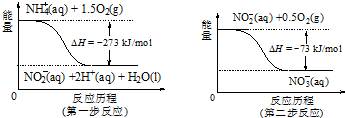
���� ��1����Ӧ2KMnO4+16HCl�T2KCl+2MnCl2+5Cl2��+8H2O�У�MnԪ�ػ��ϼ۽��ԣ�����ԭ��KMnO4Ϊ��������ClԪ�ػ��ϼ����ߣ���������HClΪ��ԭ������Ϸ�Ӧ�ķ���ʽ�����⣻
��2��������ɫ����Һ������Ӧ�ų�H2������Һ����Ϊ�������Һ��Ȼ�����ӵĹ��漰��ҺΪ���������������
��� �⣺��1����2KMnO4+16HCl�T2MnCl2+5Cl2��+8H2O+2KCl�У�MnԪ�صĻ��ϼ۽��ͣ�KMnO4Ϊ��������ClԪ�صĻ��ϼ����ߣ�������������������ΪCl2��
�ʴ�Ϊ��KMnO4��Cl2��
��n��Cl2��=$\frac{71g}{71g/mol}$=1mol��������HCl�����ʵ���Ϊ$\frac{16}{5}$mol=3.2mol����������HCl��2mol��ת�Ƶ��ӵ����ʵ����� 2mol��
�ʴ�Ϊ��3.2��2��2mol��
��2����Al���ᷴӦ����Ӧ����������������ɫ����Һ������Ӧ�ų�H2������Һ����Ϊ�������Һ��
��һ�����������ҺΪ�ᣬһ�����ڴ�����H+����H+�ֱܷ���OH-��CO32-�����ӷ�Ӧ�����ܴ������棬����OH-��SO32-��CO32-���ӣ�
�ٸ�����ҺΪ�����ԣ���һ�����ڵ�������ΪCl-��
K+��Mg2+������H+��Cl-��Ӧ���Ƿ������ȷ���������ܴ���K+��Mg2+��
�ʴ�Ϊ��H+��Cl-��K+��Mg2+��
�ڶ������������ҺΪ�һ�����ڴ�����OH-����OH-��Mg2+��H+��Ӧ�����ܴ������棬����Mg2+��H+��
�ٸ�����ҺΪ�����ԣ���һ�����ڵ�������ΪK+��OH-��K+������Cl-��SO32-��CO32-��Ӧ���Ƿ������ȷ����
��������Cl-��CO32-��NO3-��
�ʴ�Ϊ��OH-��K+��Cl-��SO32-��CO32-
���� ���⿼��������ԭ��Ӧ�Լ���Al��Ӧ������������Һ�����ӵĹ������⣬������ѧ���ķ��������ͼ��������Ŀ��飬Ϊ��Ƶ���㣬ע�����������ԭ��Ӧ����������Ԫ�ػ��ϼ۵ĽǶ���ʶ������ԭ��Ӧ����ظ�������ʵ����ʣ��ѶȲ���

| A�� | pH��7���� c��OH-����c��Na+����c��H+����c��CH3COO-�� | B�� | pH��7���� c��Na+��+c��H+��=c��OH-��+c��CH3COO-�� | ||
| C�� | pH��7����c��CH3COO-����c��H+����c��Na+����c��OH-�� | D�� | pH=7����c��CH3COO-��=c��Na+�� |
| A�� | ���Ӱ뾶��Cl-��S2-��As3- | B�� | ���ԣ�Ba��OH��2��Ca��OH��2��KOH | ||
| C�� | ���ȶ��ԣ�HCl��HBr��HI | D�� | �е㣺AsH3��PH3��NH3 |
��1��Ϊ̽�����������I2�������ʵ�Ӱ�죬������������ʵ�飬��д���еĿհ״���
| ��� | ʵ��Ŀ�� | ��Ӧ�� | ��Ӧǰ ��Һ��pH | �¶� |
| 1 | ������ | O3+NaI+H2SO4 | 5.2 | 25�� |
| 2 | ��̽��FeCl2�Է�Ӧ���ʵ�Ӱ�� | O3+NaI+H2SO4+FeCl2 | 5.2 | ��25�� |
| 3 | ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� | O3+NaI+H2SO4 | ��5.2 | 5�� |
��֪����H2S2O3��һ�������2Na2S2O3+I2�TNa2S4O6+2NaI
�ٸõζ�ʵ���г��ձ�����ƿ�⣬����Ҫ�IJ��������Ǽ�ʽ�ζ��ܣ�
�ڸ�ʵ���п�ѡ�õ��ۣ����������ƣ���ָʾ����
�۷�Ӧ����Һ�е�ĺ���Ϊ6.35cV g•L-1��
�ܼ�ͬѧ��Ϊ�ڵζ�ǰӦ����Һ�����ų���Һ���ܽ��O3��O2����Ȼ��ʹ�ζ����ƫ�ߣ���͡��ߡ�����
| A�� | ���ˮ�еμ�CCl4�����ú�ֲ㣬CCl4����Ϻ�ɫ��������˵������CCl4�ӵ�ˮ����ȡ�⣮ | |
| B�� | ��ijˮ��Һ�еμ������ữ��BaCl2��Һ���а�ɫ�������ɣ�˵��ԭ��Һ��һ������SO42-���� | |
| C�� | �������Ȼ�����Һ��У����Ƶ������������� | |
| D�� | ϡ��Ũ����ʱ��Ũ�������ձ�������ע��ˮ�в����Ͻ��� |