��Ŀ����
9����1����ϵͳ��������д�����л�������ƣ� ��������3��4һ�������飬
��������3��4һ�������飬��2��2��6-����-4-�һ�����Ľṹ��ʽ��
 ��1mol������ȫȼ������������18.5mol��
��1mol������ȫȼ������������18.5mol����3��1gCH4ȼ��ʱ����CO2��Һ̬H2O���ų�55.6 kJ��������д�������ȼ�����Ȼ�ѧ����ʽCH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��4��д������ȼ�ϵ���ڼ��������µĸ�����Ӧ��CH4-8e-+10OH-=CO32-+7H2O��
���� ��1�����л���Ϊ��������������������ԭ��Ը��л������������
��2����������������ԭ��д�����л���Ľṹ��ʽ�����ݸ��л���Ľṹ��ʽ�γɷ���ʽ��Ȼ��������ȫȼ�����ĵ����������ʵ�����
��3�������Ȼ�ѧ����ʽ����д������֪����ѧ�������뷴Ӧ�ȳ����ȣ���ע��������ʵľۼ�״̬�����ע��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų���������
��4������ȼ�ϵ�ؼ����ڸ���ʧ���ӣ��ڼ���Һ������̼������ӣ����ݵ���غ���ƽ��д�缫��Ӧ��
��� �⣺��1��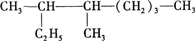 �����л����̼��8��C������Ϊ���飬��Ŵ����·���ʼ�����л�������Ϊ��3��4һ�������飬
�����л����̼��8��C������Ϊ���飬��Ŵ����·���ʼ�����л�������Ϊ��3��4һ�������飬
�ʴ�Ϊ��3��4һ�������飻
��2��2��6-����-4-�һ����飬����Ϊ���飬��2��6��C������1��������4��C����1���һ������л���ṹ��ʽΪ�� ��������ṹ��ʽ��֪���л������ʽΪ��C12H26��1mol���л�����ȫȼ�����ĵ����������ʵ���Ϊ����12+$\frac{26}{4}$��mol=18.5mol��
��������ṹ��ʽ��֪���л������ʽΪ��C12H26��1mol���л�����ȫȼ�����ĵ����������ʵ���Ϊ����12+$\frac{26}{4}$��mol=18.5mol��
�ʴ�Ϊ�� ��18.5��
��18.5��
��3��1g CH4ȼ��ʱ����CO2��Һ̬H2O���ų�55.6kJ��������ȼ������1mol��ȼ����ȫȼ�������ȶ�������ų�����������16g����ȼ�����ɶ�����̼��Һ̬ˮ����889.6kJ���Ȼ�ѧ����ʽΪ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
�ʴ�Ϊ��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=-889.6kJ/mol��
��4������ȼ�ϵ�ؼ����ڸ���ʧ���ӣ��ڼ���Һ������̼������ӣ�����ȼ�ϵ���ڼ��������µĸ�����ӦΪ��CH4-8e-+10OH-=CO32-+7H2O��
�ʴ�Ϊ��CH4-8e-+10OH-=CO32-+7H2O��
���� ���⿼�����������������Ȼ�ѧ����ʽ��д������ע�����⣬ԭ���ԭ���������缫��Ӧ��д�����ջ����ǹؼ�����Ŀ�ϼ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�| A�� | 1.6 mol SO2+0.3 mol O2+0.4 mol SO3 | |
| B�� | 4.0 mol SO2+1.0 mol O2 | |
| C�� | 2.0 mol SO2+1.0 mol O2+2.0 mol SO3 | |
| D�� | 3.0 mol SO2+1.0 mol O2+1.0 mol SO3 |
| Ԫ�� | Li | Na | K | O | O- | F |
| ��������/kJ•mol-1 | 59.8 | 52.7 | 48.4 | 141 | -780 | 327.9 |
| A�� | ��������Խ��˵��Խ���õ����� | |
| B�� | һ����̬����̬��ԭ�ӵõ�һ�����ӳ�Ϊ������ʱ�ų�327.9kJ������ | |
| C�� | Ԫ�صĵ�һ��������ͬ����Ĵ��ϵ�����С��ͬ���ڴ������������� | |
| D�� | ��̬����̬��ԭ�ӵõ��������ӳ�ΪO2-��Ҫ�������� |
| A | B | C | D | |
| ���� | Fe | C | Cu | Zn |
| ���� | Cu | Fe | Fe | Fe |
| �������Һ | CuSO4 | H2SO4 | CuCl2 | CuSO4 |
| A�� | A | B�� | B | C�� | C | D�� | D |
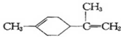 �й�����ϩ�ķ�����ȷ���ǣ�������
�й�����ϩ�ķ�����ȷ���ǣ�������| A�� | ����ϩ��һ�ȴ�����9�� | |
| B�� | ����ϩ�Ͷ�������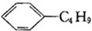 ����Ϊͬ���칹�� ����Ϊͬ���칹�� | |
| C�� | ����ϩ�ķ��������е�̼ԭ�ӿ�����ͬһ��ƽ���� | |
| D�� | һ�������£�����ϩ���Է����ӳɡ�ȡ���������ȷ�Ӧ |
| A�� | ��SiO2�����У���Si��O���ɵ���С��Ԫ���й���8��ԭ�� | |
| B�� | ��28g������У���Si-Si���ۼ�����Ϊ4NA | |
| C�� | ���ʯ���۷е���ھ���裬����ΪC-C������С��Si-Si�� | |
| D�� | þ�ͺ�ͭ�ͽ����������λ����Ϊ12 |
| A�� | 2.5 mol | B�� | 2 mol | C�� | 1.25 mol | D�� | 0.5 mol |
| A�� | 2-��-4-�һ����� | B�� | 3��4��4-�������� | ||
| C�� | 2��2��4-����-1-���� | D�� | 2��3-���һ�-1-��ϩ |