��Ŀ����
����Ŀ����������������A��B������̼���⡢���������Ⱦ�Ϊ6��1��4����ȫȼ��0.1 mol A������3.6 gˮ��Bֻ��һ��ȩ����1.1g B������������Һ��Ӧ��������2.7 g Ag�������й�������A������B���ж���ȷ����
A. ����Aһ������ȩ
B. ����A������B��Ϊͬ���칹��
C. ����B��ͬ���칹���мȺ���ȩ���ֺ����ǻ�����6��
D. ����B���������ͬ���칹����4��
���𰸡�D
��������
��������������A��B������̼���⡢���������Ⱦ�Ϊ6��1��4��C��H��Oԭ����Ŀ֮��=![]() =2:4:1���л�������ʽΪC2H4O��
=2:4:1���л�������ʽΪC2H4O��
��ȫȼ��0.1 mol A������3.6 gˮ��ˮ�����ʵ���=![]() =0.2mol����A������Hԭ����Ŀ=
=0.2mol����A������Hԭ����Ŀ=![]() =4����A�ķ���ʽΪC2H4O��
=4����A�ķ���ʽΪC2H4O��
Bֻ��һ��ȩ����1.1g B������������Һ��Ӧ��������2.7 g Ag ��Ag�����ʵ���=![]() =0.025mol���ɹ�ϵʽΪ��R-CHO~2Ag������֪��1.1g B�����ʵ���=0.025mol
=0.025mol���ɹ�ϵʽΪ��R-CHO~2Ag������֪��1.1g B�����ʵ���=0.025mol ![]() =0.0125mol ��B����Է�������=
=0.0125mol ��B����Է�������=![]() =88����B����ʽΪ��C2H4O��n����44n=88����n=2����B�ķ���ʽΪ��C4H8O2���ݴ˽��
=88����B����ʽΪ��C2H4O��n����44n=88����n=2����B�ķ���ʽΪ��C4H8O2���ݴ˽��
A.A�ķ���ʽΪC2H4O������Ϊ��ȩ��Ҳ����Ϊ�������飬��A����
B.��������������֪��������A������B�ķ���ʽ��ͬ�����߲���Ϊͬ���칹�壬��B����
C. B�ķ���ʽΪ��C4H8O2������һ��-CHO���������ǻ������Կ������鱻-CHO��-OHȡ������B���ܵĽṹ��ʽ�У�HOCH(CH2CH3)CHO��HOCH2CH(CH3)CHO��HOCH2CH2CH2CHO��(CH3)2C(OH)CHO��CH3CH(OH)CH2CHO��5�֣���C����
D. B�ķ���ʽΪ��C4H8O2�����������ͬ���칹����HCOOCH2CH2CH3��HCOOCH��CH3��2��CH3COOCH2CH3��CH3CH2COOCH3��4�֣���D��ȷ��
��ѡD��

 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�����Ŀ��2019����Ԫ�����ڱ�����150���꣬Ԫ�����ڱ����ɣ���ѧϰ���о�������ʵ�����к���Ҫ�����á��±�ΪԪ�����ڱ���һ���֣��ش��������⡣
�� ���� | IA | 0 | ||||||
1 | �� | IIA | IIIA | IVA | VA | VIA | VIIA | |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | ||||||||
��1��Ԫ�آ١����У���������ǿ����________����Ԫ�ط��ţ���
��2���й����껯ѧ�ҽ�ѩ�屻������֯��ѡΪ��Ԫ�آߴ����ˡ���Ԫ�آߵ�ԭ�ӽṹʾ��ͼ��________�����⻯��ĵ���ʽ��_________��
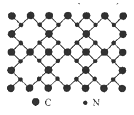
��3��Ԫ�آٺ͢ڿ����γɶ��ֻ������ͼģ�ͱ�ʾ�ķ����У��������ɢٺ͢��γɵ���_______������ţ���
��4���Ƚ�Ԫ�آڡ��۵�����������Ӧˮ��������ԣ�______��______���ѧʽ����˵�����жϵ����ɣ�_________��
��5������Ԫ���飨As���IJ�����Ϣ��ͼ��ʾ��
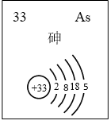
i.�飨As�������ڱ��е�λ����______��
ii.����˵����ȷ����_______������ţ���
a. ��Ԫ�ص�����ϼ�Ϊ+4
b. �Ʋ����ж���������
c. �۵���̬�⻯��Ļ�ԭ�Դ��������̬�⻯��Ļ�ԭ��
��6��ijС��ͬѧ���ʵ��Ƚ�VIIAԪ�صķǽ����ԣ�Cl>Br>I��
��֪��������Ũ�����������ط�Ӧ����������
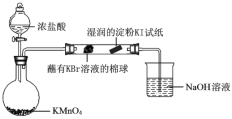
��Һ©���Ļ�������ƿ�в�������ɫ���壬պ��KBr��Һ�������Ϊ�Ⱥ�ɫ��ʪ��ĵ���KI��ֽ�������ݴ������ܷ�˵���ǽ����ԣ�Br > I����˵������_________��