��Ŀ����
 KMnO4����������pH�ļ�С�������������Խ����л�ԭ������Mn2+�������Ի���Խ����л�ԭ������Ҫ��MnO2��
KMnO4����������pH�ļ�С�������������Խ����л�ԭ������Mn2+�������Ի���Խ����л�ԭ������Ҫ��MnO2����1��Ӧ����
��2������������Һ�е�������KMnO4��Һ�����÷�Ӧ������������ԭ������ƽ���ϵ��������ȷλ�ã����������ת�Ƶķ������Ŀ
��3��������ϩ��C2HCl3���ǵ���ˮ�л���Ⱦ�����Ҫ�ɷ֣��о���ʾ�ڵ���ˮ�м���KMnO4��Һ�ɽ����е�������ϩ��ȥ����������ֻ��CO2��д����Ӧ�Ļ�ѧ����ʽ
��4�����������KMnO4���ܽ�ˮ�е�������ϩ���׳�ȥ����֪n��KMnO4����n��C2HCl3��=5��1ʱ��ˮ���е�������ϩ������ȫȥ����ij����ˮ��Ʒ��������ϩ������Ũ��Ϊ1��10-4g/L������ÿ����1m3�õ���ˮ����KMnO4
��������2������KMnO4����������pH�ļ�С�������Ʊ�KMnO4ʱӦʹ�������������ԣ�
��2�����ݻ��ϼ۱仯����������뻹ԭ��֮������غ��עת�Ƶ��ӣ�
��3��������Ŀ��Ϣ��ԭ���غ�ȷ������ٸ���������ԭ��Ӧ�е�ʧ��������Ƚ��ԭ���غ���ƽ����ʽ��
��4������������ԭ��Ӧ��n��KMnO4����n��C2HCl3��=5��1ʱ��ˮ���е�������ϩ������ȫȥ�������㣮
��2�����ݻ��ϼ۱仯����������뻹ԭ��֮������غ��עת�Ƶ��ӣ�
��3��������Ŀ��Ϣ��ԭ���غ�ȷ������ٸ���������ԭ��Ӧ�е�ʧ��������Ƚ��ԭ���غ���ƽ����ʽ��
��4������������ԭ��Ӧ��n��KMnO4����n��C2HCl3��=5��1ʱ��ˮ���е�������ϩ������ȫȥ�������㣮
����⣺��1��KMnO4����������pH�ļ�С�������Ʊ�KMnO4ʱӦʹ�������������ԣ������ڼ��Խ������Ʊ�KMnO4���ʴ�Ϊ�����ԣ�
��2������������Һ�е�������KMnO4��Һ��KMnO4�����������������ǻ�ԭ�����÷�Ӧ�л��ϼ۵ı仯Ϊ��KMnO4��MnSO4��MnԪ����+7�ۡ�+2�ۣ�һ��KMnO4���ӵ�5�����ӣ�H2SO3��H2SO4��SԪ����+4�ۡ�+6�ۣ�һ��H2SO3ʧȥ2�����ӣ���ʧ���ӵ���С������Ϊ10������KMnO4�ļ�����Ϊ2��H2SO3�ļ�����Ϊ5�����������ͻ�ԭ���Ķ�����ϵΪ��2KMnO4+5H2SO3�����ݻ��ϼ۱仯�͵����غ�õ�����ת��Ϊ10e-���ʴ�Ϊ��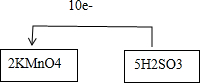 ��
��
��3��KMnO4��Һ��������ϩ��Ӧ��������Ϣ������MnO2��CO2������ԭ���غ����Ҳ�У�KCl��HCl���÷�Ӧ�л��ϼ۵ı仯Ϊ��KMnO4��MnO2��MnԪ����+7�ۡ�+4�ۣ�һ��KMnO4���ӵ�3�����ӣ�C2HCl3��CO2��CԪ����+1�ۡ�+4�ۣ�һ��C2HCl3ʧȥ6�����ӣ���ʧ���ӵ���С������Ϊ6������KMnO4�ļ�����Ϊ2��H2SO3�ļ�����Ϊ1�����ԭ���غ���ƽ����ʽ�ã�C2HCl32KMnO4+C2HCl3�T2KCl+2CO2��+2MnO2+HCl���ʴ�Ϊ��2KMnO4+C2HCl3�T2KCl+2CO2��+2MnO2+HCl��
��4���ɹ�ϵʽ��5KMnO4 ��C2HCl3
790 131.5
m ��KMnO4�� 1000L��1��10-4g/L
=
����� m ��KMnO4��=0.6 g���ʴ�Ϊ��0.6��
��2������������Һ�е�������KMnO4��Һ��KMnO4�����������������ǻ�ԭ�����÷�Ӧ�л��ϼ۵ı仯Ϊ��KMnO4��MnSO4��MnԪ����+7�ۡ�+2�ۣ�һ��KMnO4���ӵ�5�����ӣ�H2SO3��H2SO4��SԪ����+4�ۡ�+6�ۣ�һ��H2SO3ʧȥ2�����ӣ���ʧ���ӵ���С������Ϊ10������KMnO4�ļ�����Ϊ2��H2SO3�ļ�����Ϊ5�����������ͻ�ԭ���Ķ�����ϵΪ��2KMnO4+5H2SO3�����ݻ��ϼ۱仯�͵����غ�õ�����ת��Ϊ10e-���ʴ�Ϊ��
 ��
����3��KMnO4��Һ��������ϩ��Ӧ��������Ϣ������MnO2��CO2������ԭ���غ����Ҳ�У�KCl��HCl���÷�Ӧ�л��ϼ۵ı仯Ϊ��KMnO4��MnO2��MnԪ����+7�ۡ�+4�ۣ�һ��KMnO4���ӵ�3�����ӣ�C2HCl3��CO2��CԪ����+1�ۡ�+4�ۣ�һ��C2HCl3ʧȥ6�����ӣ���ʧ���ӵ���С������Ϊ6������KMnO4�ļ�����Ϊ2��H2SO3�ļ�����Ϊ1�����ԭ���غ���ƽ����ʽ�ã�C2HCl32KMnO4+C2HCl3�T2KCl+2CO2��+2MnO2+HCl���ʴ�Ϊ��2KMnO4+C2HCl3�T2KCl+2CO2��+2MnO2+HCl��
��4���ɹ�ϵʽ��5KMnO4 ��C2HCl3
790 131.5
m ��KMnO4�� 1000L��1��10-4g/L
| 790 |
| m (KMnO 4) |
| 131.5 |
| 1000L��1��10-4g/L |
������������Ҫ������������ԭ��Ӧ�е��ӵ�ʧ�غ��Լ���ѧ���㣬�Ѷ��еȣ����ݵ��ӵ�ʧ�����������ԭ��ƽ�����ݣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

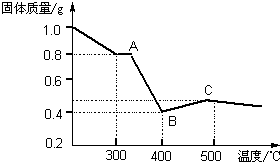 [�����о�]�������������в��ĵ���FeC2O4?2H2O���ȷֽ�ʱ�������������¶ȱ仯��������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4?2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��
[�����о�]�������������в��ĵ���FeC2O4?2H2O���ȷֽ�ʱ�������������¶ȱ仯��������ͼ��ʾ��д�����ȵ�400��ʱ��FeC2O4?2H2O�������ȷֽ�Ļ�ѧ����ʽΪ��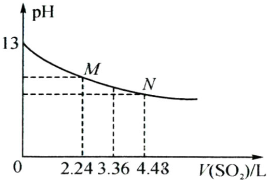
 KMnO4����������pH�ļ�С�������������Խ����л�ԭ������Mn2+�������Ի���Խ����л�ԭ������Ҫ��MnO2��
KMnO4����������pH�ļ�С�������������Խ����л�ԭ������Mn2+�������Ի���Խ����л�ԭ������Ҫ��MnO2��