��Ŀ����
1��ʵ��������NaOH�������������480mL 1.0mol/L��NaOH��Һ��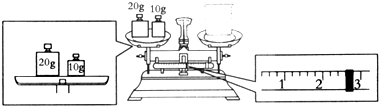
��1������ʱ������ʹ�õIJ���������500mL������ƿ���ձ�������������ͷ�ιܣ�
��2��Ҫ��ɱ�ʵ���ͬѧӦ�Ƴ�NaOH20.0g��
��3��ijͬѧ������NaOH��������������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ27.4g��
��4��ʹ������ƿǰ������е�һ�������Ǽ���Ƿ�©ˮ��
���� ��1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2��������480mL����ƿ������ѡ��500mL����ƿ�����Ƴ�500mL��Һ������m=CVM�����㣻
��3��������ƽ�ij���ԭ����������
��4������ƿ��ʹ��ǰҪ�Ȳ�©��
��� �⣺��1��û��480mL����ƿ������ѡ��500mL����ƿ�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬�ò��������裬�����ܽ⣬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�������Ҫ�IJ����������ձ�����������500mL������ƿ����ͷ�ιܣ��ʻ���������У�
500mL������ƿ�����������ձ�����ͷ�ιܣ�
�ʴ�Ϊ��500mL������ƿ���ձ�������������ͷ�ιܣ�
��2��������480mL����ƿ������ѡ��500mL����ƿ�����Ƴ�500mL��Һ����������������Ƶ�����m=CVM=1.0mol/L��0.5L��40g/mol=20.0g���ʴ�Ϊ��20.0��
��3������ƽ�ij���ԭ�����������������=�������������+����Ķ����������ձ���ʵ������Ϊ30g-2.6g=27.4g���ʴ�Ϊ��27.4��
��4������ƿ�л���������ʹ��ǰҪ�ȼ���Ƿ�©ˮ���ʴ�Ϊ������Ƿ�©ˮ��
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ��������Ϥ����ԭ���������ǽ���ؼ���ע������ƿ����ѡ����Ŀ�ѶȲ���

 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�| A�� | ʵ�����ô���ʯ��ϡ������ȡCO2��2H++CO32-=CO2��+H2O | |
| B�� | ��������������Һ��ϡ���ᷴӦ��Ca��OH��2+2H+=Ca2++2H2O | |
| C�� | ������������Һ�м�������С�մ�Ba2++2OH-+2HCO3-=BaCO3��+CO32-+2H2O | |
| D�� | NaHCO3��Һ��NaOH��Һ��Ӧ��OH-+HCO3-=CO32-+H2O |
| A�� | 1mol��������ֻ���ڱ�״���²�Լ��22.4L | |
| B�� | ��������N2��N4���ڲ�ͬ�����£�������ԭ������ͬ | |
| C�� | 22gij���庬������Ϊ0.5NA������Ħ������Ϊ44 | |
| D�� | ��״����1.12L CO��N2�Ļ�����庬��Լ6.02��1022��ԭ�� |
| ʵ����Ŀ | ʵ������ |
| FeCl3��Һ�м���ά����C��Vc�� | ��Һ��Ϊdz��ɫ |
| Fe��OH��3����ֱͨ���� | �����������ɫ���� |
| ��ͭ����������� | ����ɫ���ݲ��� |
| A�� | ά����C��Vc�����л�ԭ�� | B�� | Fe��OH��3�������Ӵ������ | ||
| C�� | ͭ�������ᷴӦһ����CO2���� | D�� | ͭ���п��ܺ���̼��� |

�������ʵ��й��������£�
| ��ѧʽ | N2 | O2 | CO2 | NH3 | Cl2 |
| �۵㣨�棩 | -209.86 | -218.4 | -78.5 | -77.3 | -101 |
| �е㣨�棩 | -195.8 | -183 | -33.35 | -34.6 |
��1����˵�������Լ�ǰ����A��B��C�����������ԵIJ��������ȹرշ�Һ©���ϵĻ������رտ���N�Ļ�������M�ܿڲ���ʢ��ˮ��ˮ���У���A����Բ����ƿ�ȣ����ܿ�M��������ð����ֹͣ����M����Һ��������֤��A��B��C����װ�����������ã�
��2��A��ʢ�е���ɫ�����Լ�aӦ�Ǹ�����أ���Һ©���е�b�Լ���Ũ���ᣮ
��3��B��ʢ�е�Һ��CӦ�DZ���ʳ��ˮ��C�е�Һ��d��Ũ���ᣮ
��4��D�з�����Ӧ�Ļ�ѧ����ʽ��2HgO+2Cl2=Cl2O+HgO•HgCl2��
��5��E�еı���ƿ��ʢ��Һ̬�����c����Ӧ��Һ̬�������ڡ��ɱ���������ˮ������Һ̬����������Һ��������Һ�ȡ���ѡ��һ�֣�����E���ڹܵõ���Cl2O�п��ܺ��е�������Ҫ��Һ�ȣ�
��6��װ��A��B��C������ӷ�ʽ��D��E������ӷ�ʽ�����ԵIJ����������A��B��C�����齺�����ӣ�D��E�䲻���齺�����ӣ������ֲ�ͬ�����ӷ�ʽ����Ҫ������Cl2O���л����ױ�ը��