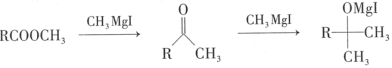
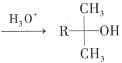
��Ŀ����
����Ŀ�����ռ�������ˮ�����ξ��ơ���һ�ξ�����Ҫ���ó�������ȥ����ˮ��Ca2+��Mg2+��Fe3+��SO![]() �����ӣ��������£�
�����ӣ��������£�
��. �����ˮ�м������BaCl2��Һ�����ˣ�
��. ��������Һ�м������Na2CO3��Һ�����ˣ�
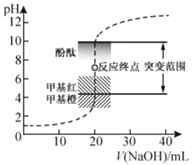
��. ��Һ���������pH�����һ�ξ�����ˮ��
(1)��������ȥ��������______��
(2)�������������ɵIJ��ֳ��������ܽ��(20��/g)���±���
�ټ��Fe3+�Ƿ�����ķ�����______��
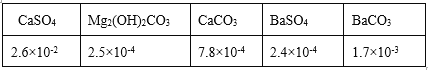
�ڹ�����ѡ��BaCl2����ѡ��CaCl2�����ñ������ݽ���ԭ��______��
�۳�ȥMg2+�����ӷ���ʽ��______��
�ܼ��Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ�ԭ����_____��
(3)�ڶ��ξ���Ҫ��ȥ����I-��IO![]() ��NH
��NH![]() ��Ca2+��Mg2+������ʾ�����£�
��Ca2+��Mg2+������ʾ�����£�
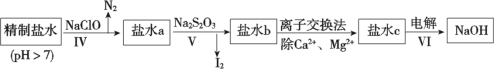
�� ��������ȥ��������______��
�� ��ˮb�к���SO![]() ��Na2S2O3��IO
��Na2S2O3��IO![]() ��ԭΪI2�����ӷ���ʽ��________ ��
��ԭΪI2�����ӷ���ʽ��________ ��
�� ����VI�У��ڵ��۵�����������Ӧ�ĵ缫����ʽ�ǣ�_________________��
���𰸡�SO![]() ȡ��������II�����Һ���Թ��У��μӼ���KSCN ��Һ������Һ����죬˵��Fe 3+�ѳ�������֮û���� BaSO4���ܽ�ȱ�CaSO4��С���ɽ�SO
ȡ��������II�����Һ���Թ��У��μӼ���KSCN ��Һ������Һ����죬˵��Fe 3+�ѳ�������֮û���� BaSO4���ܽ�ȱ�CaSO4��С���ɽ�SO![]() ��������ȫ 2Mg2+ + 2CO
��������ȫ 2Mg2+ + 2CO![]() + H2O = Mg2(OH)2CO3��+ CO2�� ��BaCO3��CaCO3��Mg2(OH)2CO3 �У�BaCO3���ܽ�������Ba2+������ȫ����˵��Mg2+ ��Ca2+Ҳ������ȫ NH
+ H2O = Mg2(OH)2CO3��+ CO2�� ��BaCO3��CaCO3��Mg2(OH)2CO3 �У�BaCO3���ܽ�������Ba2+������ȫ����˵��Mg2+ ��Ca2+Ҳ������ȫ NH![]() ��I- 5S2O
��I- 5S2O![]() + 8IO
+ 8IO![]() + 2OH- =4I2 + 10SO
+ 2OH- =4I2 + 10SO![]() + H2O 2 Cl -- 2e- = Cl2��
+ H2O 2 Cl -- 2e- = Cl2��
��������
��һ�ξ��ƣ������ˮ�м������BaCl2��Һ��Ba2+��SO![]() �õ�BaSO4���������ˣ���ȥBaSO4��������������Һ�м������Na2CO3��Һ����ȥCa2+��Mg2+��Fe3+������Ba2+�����˺����������pH����ȥ������̼���ƣ��õ�������ˮ����ϱ������ݷ������(1)��(2)��
�õ�BaSO4���������ˣ���ȥBaSO4��������������Һ�м������Na2CO3��Һ����ȥCa2+��Mg2+��Fe3+������Ba2+�����˺����������pH����ȥ������̼���ƣ��õ�������ˮ����ϱ������ݷ������(1)��(2)��
�ڶ��ξ��ƣ����һ�ξ�����ˮ(��������ΪI-��IO![]() ��NH
��NH![]() ��Ca2+��Mg2+)�м���NaClO��NaClO���������ԣ���I-����ΪI2��NH
��Ca2+��Mg2+)�м���NaClO��NaClO���������ԣ���I-����ΪI2��NH![]() ����ΪN2���ټ���Na2S2O3����IO
����ΪN2���ټ���Na2S2O3����IO![]() ��ԭΪI2�������I2��ͨ�����ӽ�������ȥCa2+��Mg2+�����ʣ����Һ(�����ƺ��Ȼ��ƵĻ����Һ)�õ�NaOH���ݴ˷������(3)��
��ԭΪI2�������I2��ͨ�����ӽ�������ȥCa2+��Mg2+�����ʣ����Һ(�����ƺ��Ȼ��ƵĻ����Һ)�õ�NaOH���ݴ˷������(3)��
(1)�����ˮ�м������BaCl2��Һ��Ba2+��SO![]() �õ�BaSO4���������ˣ���ȥSO
�õ�BaSO4���������ˣ���ȥSO![]() ���ʴ�Ϊ��SO
���ʴ�Ϊ��SO![]() ��
��
(2) ��Fe3+�ܹ�ʹKSCN ��Һ��죬��˼��Fe3+�Ƿ�����ķ�����ȡ��������II�����Һ���Թ��У��μӼ���KSCN ��Һ������Һ����죬˵��Fe 3+�ѳ�������֮û�������ʴ�Ϊ��ȡ��������II�����Һ���Թ��У��μӼ���KSCN ��Һ������Һ����죬˵��Fe 3+�ѳ�������֮û������
�ڸ��ݱ������ݿ�֪��BaSO4![]() ��������ȫ���ʹ�����ѡ�õ���BaCl2����ѡ��CaCl2���ʴ�Ϊ��BaSO4���ܽ�ȱ�CaSO4��С���ɽ�SO
��������ȫ���ʹ�����ѡ�õ���BaCl2����ѡ��CaCl2���ʴ�Ϊ��BaSO4���ܽ�ȱ�CaSO4��С���ɽ�SO![]() ��������ȫ��
��������ȫ��
��Mg2+��CO![]() ����Mg2(OH)2CO3�Ͷ�����̼�����ӷ���ʽΪ��2Mg2++2CO
����Mg2(OH)2CO3�Ͷ�����̼�����ӷ���ʽΪ��2Mg2++2CO![]() +H2O=Mg2(OH)2CO3��+CO2�����ʴ�Ϊ��2Mg2++2CO
+H2O=Mg2(OH)2CO3��+CO2�����ʴ�Ϊ��2Mg2++2CO![]() +H2O=Mg2(OH)2CO3��+CO2����
+H2O=Mg2(OH)2CO3��+CO2����
��Ca2+��Mg2+��Ba2+��CaCO3��Mg2(OH)2CO3��BaCO3����ʽ��ȥ�������ܽ��С�����ʳ�����ȫ���ܽ�ȴ�IJſ�ʼ�������ɱ������ݿ�֪��̼�ᱵ���ܽ������������ӳ�����ȫ����˵��þ���Ӻ�����Ҳ������ȫ���ʼ��Ca2+��Mg2+��Ba2+�Ƿ����ʱ��ֻ����Ba2+���ɣ��ʴ�Ϊ����BaCO3��CaCO3��Mg2(OH)2CO3 �У�BaCO3���ܽ�������Ba2+������ȫ����˵��Mg2+��Ca2+Ҳ������ȫ��
(3)��NaClO����ǿ�����ԣ��ܽ�I-�������������̿�֪NH![]() ������ΪN2���ʹ�������ȥ��������NH
������ΪN2���ʹ�������ȥ��������NH![]() ��I-���ʴ�Ϊ��NH
��I-���ʴ�Ϊ��NH![]() ��I-��
��I-��
��Na2S2O3��IO![]() ��ԭΪI2������������ΪSO
��ԭΪI2������������ΪSO![]() ����Ӧ�����ӷ���ʽΪ��5S2O
����Ӧ�����ӷ���ʽΪ��5S2O![]() +8IO
+8IO![]() +2OH-�T4I2+10SO
+2OH-�T4I2+10SO![]() +H2O���ʴ�Ϊ��5S2O
+H2O���ʴ�Ϊ��5S2O![]() +8IO
+8IO![]() +2OH-�T4I2+10SO
+2OH-�T4I2+10SO![]() +H2O��
+H2O��
�� ����VI�У���������ƺ��Ȼ��ƵĻ����Һ�����������ơ��������������ڵ��۵���������������Ӧ�������ĵ缫��ӦʽΪ2 Cl -- 2e- = Cl2�����ʴ�Ϊ��2 Cl- - 2e-= Cl2����