��Ŀ����
18�� 2002��ŵ������ѧ������ߵĹ���֮һ�Ƿ����˶��л�����ӽ��нṹ�������������䷽�����ü������ģ�10-9g��������ͨ�������ǵ����ӻ���ʹ��Ʒ���Ӵ������ӻ��������������ѳɸ�С�����ӣ���C2H6���ӻ���ɵõ�C2H6+��C2H5+��C2H4+����Ȼ��ⶨ���ʺɱȣ�ij�л�����Ʒ���ʺɱ�����ͼ��ʾ���������Ӿ���һ����λ����ɣ��ź�ǿ��������ӵĶ����йأ�������л�������ǣ�������
2002��ŵ������ѧ������ߵĹ���֮һ�Ƿ����˶��л�����ӽ��нṹ�������������䷽�����ü������ģ�10-9g��������ͨ�������ǵ����ӻ���ʹ��Ʒ���Ӵ������ӻ��������������ѳɸ�С�����ӣ���C2H6���ӻ���ɵõ�C2H6+��C2H5+��C2H4+����Ȼ��ⶨ���ʺɱȣ�ij�л�����Ʒ���ʺɱ�����ͼ��ʾ���������Ӿ���һ����λ����ɣ��ź�ǿ��������ӵĶ����йأ�������л�������ǣ�������| A�� | �״� | B�� | ���� | C�� | ���� | D�� | ��ϩ |
���� ��������ͼ���л�����Ʒ���ʺɱȵ����ֵΪ�����ʵ���Է���������Ȼ�������Է���������ȷ�����ʵķ���ʽ��
��� �⣺���л�����Ʒ������ͼ��֪�����л������ʺɱ����ֵΪ16������л������Է�������Ϊ16���״������顢���顢��ϩ����Է�������Ϊ32��16��42��28������л�����飬��ѡB��
���� ���⿼����������ͼȷ���л������ʽ���ѶȲ����ܹ�����ѧ������ͼ���мĶ�ͼʶ��

��ϰ��ϵ�д�
�����Ŀ
6����ͼΪH2��O2��Ӧ����H2O��g���������仯ʾ��ͼ�������й���������ȷ���ǣ�������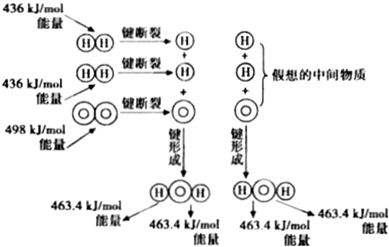

| A�� | 1molH2���Ӷϼ���Ҫ����436kJ������ | |
| B�� | H2��g��+$\frac{1}{2}{O}_{2}$��g���TH2O��g����H=-241.8kJ/mol | |
| C�� | ��Ӧ�������������������������� | |
| D�� | �γɻ�ѧ���ͷŵ��������ȶ��ѻ�ѧ�����յ�������С |
13�����ǻ�ѧʵ���Ҽ����������е���Ҫ���ʣ�Ӧ�ù㷺��
��1����֪25��ʱ��N2��g��+O2��g��?2NO��g����H=+183kJ/mol
2H2��g��+O2��g��?2H2O��l����H=-571.6kJ/mol
4NH3��g��+5O2��g��?4NO��g��+6H2O��l����H=-1164.4kJ/mol
��N2��g��+3H2��g��?2NH3��g����H=-92.2kJ/mol
��2���ں����ܱ������н��кϳɰ���Ӧ����ʼͶ��ʱ������Ũ�����±���
�ٰ�Ͷ�Ϣ���з�Ӧ����ôﵽ��ѧƽ��״̬ʱH2��ת����Ϊ40%������¶��ºϳɰ���Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��NH{\;}_{3}��{\;}^{2}}{c��H{\;}_{2}��{\;}^{3}c��N{\;}_{2}��}$��
�ڰ�Ͷ�Ϣ���з�Ӧ����ʼʱ��Ӧ���еķ���Ϊ���������������
���������¶ȣ���ϳɰ���Ӧ�Ļ�ѧƽ�ⳣ����С����������С�����䡱����
��L��L1��L2����X�ɷֱ����ѹǿ���¶ȣ���ͼ1��ʾLһ��ʱ���ϳɰ���Ӧ��H2��g����ƽ��ת������X�ı仯��ϵ��
i��X���������������¶ȣ�
ii���ж�L1��L2�Ĵ�С��ϵ������������L1��L2��������������ʱ������ѹǿ���������ƶ�������ת���ʴ�
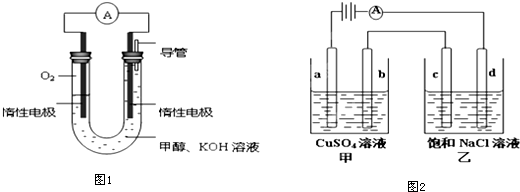
��3���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ2���£�
�ٵ缫b�Ϸ������ǻ�ԭ��Ӧ�����������ԭ������
��д���缫a�ĵ缫��Ӧʽ��2NH3-6e-+6OH-=N2+6H2O��

��1����֪25��ʱ��N2��g��+O2��g��?2NO��g����H=+183kJ/mol
2H2��g��+O2��g��?2H2O��l����H=-571.6kJ/mol
4NH3��g��+5O2��g��?4NO��g��+6H2O��l����H=-1164.4kJ/mol
��N2��g��+3H2��g��?2NH3��g����H=-92.2kJ/mol
��2���ں����ܱ������н��кϳɰ���Ӧ����ʼͶ��ʱ������Ũ�����±���
| N2 | H2 | NH3 | |
| Ͷ�Ϣ� | 1.0mol/L | 3.0mol/L | 0 |
| Ͷ�Ϣ� | 0.5mol/L | 1.5mol/L | 1.0mol/L |
�ڰ�Ͷ�Ϣ���з�Ӧ����ʼʱ��Ӧ���еķ���Ϊ���������������
���������¶ȣ���ϳɰ���Ӧ�Ļ�ѧƽ�ⳣ����С����������С�����䡱����
��L��L1��L2����X�ɷֱ����ѹǿ���¶ȣ���ͼ1��ʾLһ��ʱ���ϳɰ���Ӧ��H2��g����ƽ��ת������X�ı仯��ϵ��
i��X���������������¶ȣ�
ii���ж�L1��L2�Ĵ�С��ϵ������������L1��L2��������������ʱ������ѹǿ���������ƶ�������ת���ʴ�
��3���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ2���£�
�ٵ缫b�Ϸ������ǻ�ԭ��Ӧ�����������ԭ������
��д���缫a�ĵ缫��Ӧʽ��2NH3-6e-+6OH-=N2+6H2O��

3��ij��ɫ��ĩ������Fe3O4��Fe3O4��FeO�Ļ���Ϊ��һ��ȷ���ú�ɫ��ĩ�ijɷ֣����з��������е��ǣ�������
| A�� | ȷ����һ�������ĺ�ɫ��ĩ����H2��ֻ�ԭ�����ø�����ռ����õ�ˮ�����ˮ��ȷ���������м��� | |
| B�� | ȷ����һ�������ĺ�ɫ��ĩ���ܽ����������ᣬ����������Һ���ڿ������������������䣬�������÷�ĩ���������м��㣮 | |
| C�� | ȷ����һ�������ĺ�ɫ��ĩ����CO��ֻ�ԭ����CO��������ȴ��ȷ����ʣ���������������㣮 | |
| D�� | ȷ����һ�������ĺ�ɫ��ĩ����һ���������ۻ�Ϻ��ȼ����ַ�Ӧ����ȴ��ȷ����ʣ��������������м��� |
17�����������У����ܱ�����KMnO4��Һ���������ܺ���ˮ��Ӧ���ǣ�������
�ٱ�ϩ �ڱ�Ȳ �۱� �ܼױ� �ݾ���ϩ �ޱ��� ���������� �����ᣮ
�ٱ�ϩ �ڱ�Ȳ �۱� �ܼױ� �ݾ���ϩ �ޱ��� ���������� �����ᣮ
| A�� | �٢ۢݢ� | B�� | �٢ܢ� | C�� | �٢ڢ� | D�� | �٢ڢޢ� |


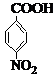 ��B
��B ��D�ĺ��������ŵ�����Ϊ������
��D�ĺ��������ŵ�����Ϊ������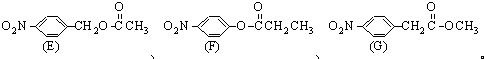
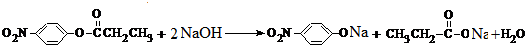 ��
��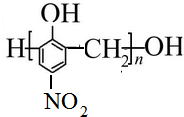 ��
��
 ��
��