��Ŀ����
7����1��1mol/L��BaCl2��Һ0.5L�У�����Ba2+����Ŀ��0.5NA����28g KOH���250mL��Һ�����ʵ����ʵ�����0.5mol����Һ�����ʵ���Ũ����2mol/L����2����20g�ռ����Ƴ�500mL��Һ�������ʵ���Ũ��Ϊ1 mol/L������ȡ��1mL�������ʵ���Ũ��Ϊ1mol/L��������0.04g��������1mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ0.01mol/L�����к�Na+0.023g��
���� ����n=cV��N=nNA���㣻����n=$\frac{m}{M}$��c=$\frac{n}{V}$���㣻��ҺΪ��һ�ȶ���ɢϵ��ȡ�������������ԭ��ҺŨ����ȣ�������Һϡ��ǰ�����ʵ����ʵ����������ϡ�ͺ����ʵ����ʵ���Ũ�ȣ�
��� �⣺��1��1mol/L��BaCl2��Һ0.5L�У�n=cV=1mol/L��0.5L=0.5mol����Ba2+�����ʵ���0.5mol������Ba2+����Ŀ��N=nNA=0.5NA��
n=$\frac{m}{M}$=$\frac{28g}{56g/mol}$=0.5mol��c=$\frac{n}{V}$=$\frac{0.5mol}{0.25L}$=2mol/L��
�ʴ�Ϊ��0.5NA��0.5mol��2mol/L��
��2��n=$\frac{m}{M}$=$\frac{20g}{40g/mol}$=0.5mol��c=$\frac{n}{V}$=$\frac{0.5mol}{0.5L}$=1mol/L������ȡ��1mL�������ʵ���Ũ�Ȳ��䣬Ϊ1mol/L��
n=cV=0.001L��1mol/L=0.001mol��m=nM=0.001mol��40g/mol=0.04g��
��Һϡ��ǰ�����ʵ����ʵ������䣬��1mL��Һ��ˮϡ�͵�100mL��������Һ�����ʵ����ʵ���Ũ��Ϊ$\frac{0.001L��1mol/L}{0.1L}$=0.01mol/L��
���к�Na+�����ʵ���Ϊ��0.01mol/L��0.1L=0.001mol������Ϊ0.001mol��23g/mol=0.023g��
�ʴ�Ϊ��1��1��0.04��0.01��0.023��
���� ���⿼�����ʵ���Ũ�ȼ��㣬��Ŀ�ѶȲ���ע���йؼ��㹫ʽ�����ã�������Һ���ص��Լ���Һϡ��ǰ�����ʵ����ʵ��������������

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�| A�� | CH3Cl��CH2Cl2��CHCl3ˮ������ղ��ﶼ��CH3OH | |
| B�� | 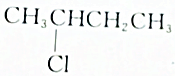 ������ȥ��Ӧ�IJ��ﲻֻһ�� ������ȥ��Ӧ�IJ��ﲻֻһ�� | |
| C�� | ���屽���뵽AgNO3��Һ�У������е���ɫ�������� | |
| D�� | 1��2-����������NaOH��ˮ��Һ�й��ȿɵõ���Ȳ |
| A�� | ���� | B�� | ��ȡ | C�� | ���� | D�� | ���� |
| A�� | FeCl3ϡ��Һ | B�� | NaClϡ��Һ | C�� | ����Ca��OH��2��Һ | D�� | ����NaHCO3��Һ |
| A�� | Na2CO3$\frac{\underline{\;ͨ��\;}}{\;}$2Na++CO32- | B�� | MgSO4�TMg2++SO42- | ||
| C�� | NaHCO3�TNa++H++CO32- | D�� | NaHSO3$\frac{\underline{\;����\;}}{\;}$Na++H++SO42- |
| A�� | Ħ������g•mol-1 | |
| B�� | ����Ħ�����L•mol-1 | |
| C�� | ��ԭ�ӵ�Ħ�����������������ԭ������ | |
| D�� | һ����ԭ�ӵ�����Լ����$\frac{23}{6.02��1{0}^{23}}$g |