��Ŀ����
��18�֣�
��1��������FeSO4��Һʱ���������������ۣ�Ŀ����___________________________________������������H2SO4��Ŀ����__________________________________________________��
��2������ĭ��������Al2(SO4)3��Һ��Լ1 mol��L-1����NaHCO3��Һ��Լ1 mol��L-1�������ݼ���ʹ��ʱ�����Ļ�ѧ��Ӧ�����ӷ���ʽ��_____________________________��
(3)����֪ Ϊ������ˮ����İ�ɫ���壬
Ϊ������ˮ����İ�ɫ���壬 Ϊ������ˮ�����Ǻ�ɫ���塣
Ϊ������ˮ�����Ǻ�ɫ���塣 ��
��
ˮ������Һ�м��������� ��Һ���������ɫ������ȫת��Ϊ��ɫ���塣
��Һ���������ɫ������ȫת��Ϊ��ɫ���塣
�١�д����ɫ����ת����ɫ����Ļ�ѧ����ʽ�� ��
�ڡ���ɫ����ת���ɺ�ɫ�����ԭ���ǣ� ��
��4������֪����������20���µ�Ksp���£��Իش��������⣺
��20��ʱ�������������α�����Һ�У�Ag�����ʵ���Ũ���ɴ�С��˳���ǣ�����ţ�
_____________________________��
����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ����_______________ ��
��1��������FeSO4��Һʱ���������������ۣ�Ŀ����___________________________________������������H2SO4��Ŀ����__________________________________________________��
��2������ĭ��������Al2(SO4)3��Һ��Լ1 mol��L-1����NaHCO3��Һ��Լ1 mol��L-1�������ݼ���ʹ��ʱ�����Ļ�ѧ��Ӧ�����ӷ���ʽ��_____________________________��
(3)����֪
 Ϊ������ˮ����İ�ɫ���壬
Ϊ������ˮ����İ�ɫ���壬 Ϊ������ˮ�����Ǻ�ɫ���塣
Ϊ������ˮ�����Ǻ�ɫ���塣 ��
�� ˮ������Һ�м���������
 ��Һ���������ɫ������ȫת��Ϊ��ɫ���塣
��Һ���������ɫ������ȫת��Ϊ��ɫ���塣 �١�д����ɫ����ת����ɫ����Ļ�ѧ����ʽ�� ��
�ڡ���ɫ����ת���ɺ�ɫ�����ԭ���ǣ� ��
��4������֪����������20���µ�Ksp���£��Իش��������⣺
| ��ѧʽ | �� AgCl | �� AgBr | �� Ag2S | �� Ag2CrO4 |
| Ksp | 2.0��10��10 | 5.4��10��13 | 2.0��10��48 | 2.0��10��12 |
_____________________________��
����BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ����_______________ ��
��1����ֹFe2+������ (2��) ����ֹFe2+ˮ�⡣(2��)��
��2��Al3++ 3HCO3- =Al(OH)3 ��+ 3CO2�� (3��) (3) ��2AgCl+Na2S==Ag2S+2NaCl��3�֣���
����ΪAg2S���ܽ�ȱ�AgCl���ܽ��С�������ܽ�ƽ��������Ũ�ȼ�С�ķ����ƶ�����2�֣�
(4) �١��� >��>��>�� ��3�֣��� �ڡ�2.7��10��3����3�֣�
��2��Al3++ 3HCO3- =Al(OH)3 ��+ 3CO2�� (3��) (3) ��2AgCl+Na2S==Ag2S+2NaCl��3�֣���
����ΪAg2S���ܽ�ȱ�AgCl���ܽ��С�������ܽ�ƽ��������Ũ�ȼ�С�ķ����ƶ�����2�֣�
(4) �١��� >��>��>�� ��3�֣��� �ڡ�2.7��10��3����3�֣�
��1�����������ױ��������������ӣ��������ۿ��Է�ֹFe2+����������������ˮ�����ԣ�����������Է�ֹFe2+ˮ�⡣
��2��������ˮ�������ԣ�̼������ˮ���Լ��ԣ�������ٽ�����������������CO2������ʽΪAl3++ 3HCO3- =Al(OH)3 ��+ 3CO2����
��3����ΪAg2S���ܽ�ȱ�AgCl���ܽ��С�������ܽ�ƽ��������Ũ�ȼ�С�ķ����ƶ��������Ȼ�����ת��Ϊ����������ʽΪ2AgCl+Na2S==Ag2S+2NaCl��
��4���ٸ����������ʵ��ܶȻ������Լ���ѧʽ��֪��������Һ��������Ũ�ȷֱ���1.4��10��5mol/L��7.3��10��7mol/L��1.6��10��16mol/L��1.6��10��4mol/L��������Һ��������Ũ�ȴ�С˳��Ϊ�� >��>��>�ۡ�
�ڸ�������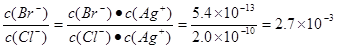
��2��������ˮ�������ԣ�̼������ˮ���Լ��ԣ�������ٽ�����������������CO2������ʽΪAl3++ 3HCO3- =Al(OH)3 ��+ 3CO2����
��3����ΪAg2S���ܽ�ȱ�AgCl���ܽ��С�������ܽ�ƽ��������Ũ�ȼ�С�ķ����ƶ��������Ȼ�����ת��Ϊ����������ʽΪ2AgCl+Na2S==Ag2S+2NaCl��
��4���ٸ����������ʵ��ܶȻ������Լ���ѧʽ��֪��������Һ��������Ũ�ȷֱ���1.4��10��5mol/L��7.3��10��7mol/L��1.6��10��16mol/L��1.6��10��4mol/L��������Һ��������Ũ�ȴ�С˳��Ϊ�� >��>��>�ۡ�
�ڸ�������

��ϰ��ϵ�д�
�����Ŀ