题目内容
【题目】某同学欲验证碳与浓硫酸反应产物的性质。现已将装置如图连接,请回答下列问题。
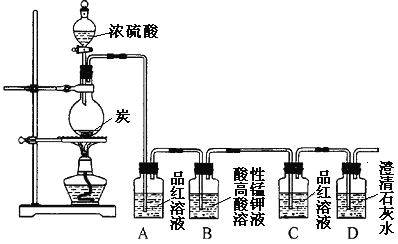
(1)烧瓶中发生反应的化学反应方程式是___________。
(2)实验中两次使用到品红溶液,其目的不同。A的使用目的是_____,通过洗气瓶C中无现象和___的现象,证明反应有_______(填化学式)生成。
(3)洗气瓶B中溶液颜色变浅,说明碳与浓硫酸反应的产物之一______(填名称)具有______的性质。
(4)实验完成后,取出洗气瓶A中的无色溶液于试管中,加热,可观察到__________。
【答案】C+2H2SO4(浓)![]() CO2↑+2SO2↑+2H2O 检验反应中有SO2气体生成 澄清石灰水变浑浊 CO2 SO2 还原性 无色溶液又恢复红色
CO2↑+2SO2↑+2H2O 检验反应中有SO2气体生成 澄清石灰水变浑浊 CO2 SO2 还原性 无色溶液又恢复红色
【解析】
浓硫酸的强氧化性,加热条件下能够将木炭氧化成生成CO2、SO2和水,将反应后的混合气体依次通过A装置,品红褪色,说明有SO2气体生成,通过B中酸性高锰酸钾溶液,利用SO2的还原性,除去SO2,再通过C中品红时,品红不褪色,说明SO2已经完全除去,再通过澄清石灰水,石灰水变浑浊,说明有CO2气体生成。
(1)浓硫酸具有强氧化性,在加热的条件下,能把木炭氧化生成CO2、SO2和水,反应的化学方程式为:C+2H2SO4(浓)![]() CO2↑+2SO2↑+2H2O;
CO2↑+2SO2↑+2H2O;
(2)A中品红褪色,可检验反应中有SO2气体生成;经过B装置中酸性高锰酸钾溶液除去SO2后,可通过洗气瓶C中品红不褪色和D中澄清石灰水变浑浊,证明反应有CO2生成;
(3)洗气瓶B中酸性高锰酸钾溶液颜色变浅,说明碳与浓硫酸反应的产物之一SO2具有还原性;
(4)因SO2与品红生成了不稳定的无色物质,则A中品红溶液褪色后,再加热,溶液又恢复红色。

 快乐小博士巩固与提高系列答案
快乐小博士巩固与提高系列答案【题目】乙酸乙酯广泛用于药物、燃料、香料等工业,在中学化学实验室里常用如图装置来制备乙酸乙酯。![]() 部分夹持仪器已略去
部分夹持仪器已略去![]()
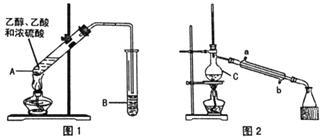
已知:
密度(g/cm3) | 熔点(℃) | 沸点(℃) | 溶解度 | |
乙醇 | 0.79 | -114.5 | 78.4 | 与水互溶 |
乙酸 | 1.05 | 16.6 | 118.1 | 易溶于水、乙醇 |
乙酸乙酯 | 0.90 | -83.6 | 77.2 | 微溶于水,能溶于乙醇 |
Ⅰ制备粗品(图1)
在A中加入少量碎瓷片,将三种原料依次加入A中,用酒精灯缓慢加热,一段时间后在B中得到乙酸乙酯粗品。
(1)浓硫酸、乙醇、乙酸的加入顺序是___,A中发生反应的化学方程式是___。
(2)A中碎瓷片的作用是___,长导管除了导气外,还具有的作用是___。
(3)B中盛装的液体是___,收集到的乙酸乙酯在___层(填“上”或“下”)。
Ⅱ.制备精品(图2)
将B中的液体分液,对乙酸乙酯粗品进行一系列除杂操作后转移到C中,利用图2装置进一步操作即得到乙酸乙酯精品。
(4)C的名称是___。
(5)实验过程中,冷却水从___口进入(填字母);收集产品时,控制的温度应在___左右。
【题目】二氧化氮可由NO和O2生成,已知在2L密闭容器内,800℃时反应:2NO(g)+O2(g)![]() 2NO2(g) ΔH,n(NO)、n(O2)随时间的变化如表:
2NO2(g) ΔH,n(NO)、n(O2)随时间的变化如表:
时间/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)/mol | 0.200 | 0.100 | 0.080 | 0.050 | 0.050 | 0.050 |
n(O2)/mol | 0.100 | 0.050 | 0.040 | 0.025 | 0.025 | 0.025 |
(1)已知:K800℃>K1000℃,则该反应的ΔH___0(填“大于”或“小于”),用O2表示0~2 s内该反应的平均速率为___。
(2)能说明该反应已达到平衡状态的是___。
a.容器内气体颜色保持不变 b.2v逆(NO)=v正(O2)
c.容器内压强保持不变 d.容器内气体密度保持不变
(3)为使该反应的速率增大,提高NO的转化率,且平衡向正反应方向移动,应采取的措施有_____。
(4)在题述条件下,计算通入2molNO和1molO2的平衡常数K=___。
(5)在题述条件下,若开始通入的是0.2molNO2气体,达到化学平衡时,NO2的转化率为__。
(6)煤燃烧产生的烟气含氮的氧化物,用CH4催化还原NOx可以消除氮氧化物的污染。
①CH4(g)+4NO(g)![]() 2N2(g)+CO2(g)+2H2O ΔH<0
2N2(g)+CO2(g)+2H2O ΔH<0
②CH4(g)+2NO2(g)![]() N2(g)+CO2(g)+2H2O(g) ΔH<0
N2(g)+CO2(g)+2H2O(g) ΔH<0
对于反应②,欲提高NO2的转化率,可采取的措施有____。
a.增加原催化剂的表面积 b.降低温度 c.减小投料比[n(NO2)/n(CH4)] d.增大压强
【题目】催化还原CO2是解决温室效应及能源问题的重要手段之一。研究表明,在Cu/ZnO催化剂存在下,CO2和H2可发生两个平行反应,分别生成CH3OH和CO。反应的热化学方程式如下:
CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ΔH1=-53.7kJ·mol-1 I
CH3OH(g)+H2O(g) ΔH1=-53.7kJ·mol-1 I
CO2(g)+H2(g)![]() CO(g)+H2O(g) ΔH2 II
CO(g)+H2O(g) ΔH2 II
某实验室控制CO2和H2初始投料比为1:2.2,在相同压强下,经过相同反应时间测得如下实验数据:
T(K) | 催化剂 | CO2转化率(%) | 甲醇选择性(%) |
543 | Cat.1 | 12.3 | 42.3 |
543 | Cat.2 | 10.9 | 72.7 |
553 | Cat.1 | 15.3 | 39.1 |
553 | Cat.2 | 12.0 | 71.6 |
(备注)Cat.1:Cu/ZnO纳米棒;Cat.2:Cu/ZnO纳米片;甲醇选择性:转化的CO2中生成甲醇的百分比
已知:①CO和H2的标准燃烧热分别为-283.0kJ·mol-1和-285.8kJ·mol-1
②H2O(l)=H2O(g) ΔH3=44.0kJ·mol-1
请回答(不考虑温度对ΔH的影响):
(1)反应I的平衡常数表达式K=___;
(2)有利于提高CO2转化为CH3OH平衡转化率的措施有___。
A.使用催化剂Cat.1
B.使用催化剂Cat.2
C.降低反应温度
D.投料比不变,增加反应物的浓度
E.增大CO2和H2的初始投料比
(3)表中实验数据表明,在相同温度下不同的催化剂对CO2转化成CH3OH的选择性有显著的影响,其原因是___。
(4)在图中分别画出反应I在无催化剂、有Cat.1和有Cat.2三种情况下“反应过程~能量”示意图___。