��Ŀ����
����Ŀ���о�NOx��CO�ȴ�����Ⱦ��IJ���������������Ҫ���塣
��1��������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�NOx�ڴ�ת�����б�CO��ԭ��N2�ų���д��NO��CO��ԭ�Ļ�ѧ����ʽ��________________��
��2��ѡ���Դ���ԭ����(SCR)��Ŀǰ�����������������������ڽ������������£��û�ԭ��(��NH3)ѡ���Ե���NOx��Ӧ����N2��H2O��
����֪��4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)��H=-905.5kJmol-1
4NO(g)+6H2O(g)��H=-905.5kJmol-1
N2(g)+O2(g)![]() 2NO(g)��H=+180kJmol-1
2NO(g)��H=+180kJmol-1
��ɸ÷�������Ҫ��Ӧ���Ȼ�ѧ����ʽ
4NH3(g)+4NO(g)+O2(g)![]() 4N2(g)+6H2O(g)��H=_________________��
4N2(g)+6H2O(g)��H=_________________��
�ڸ÷���Ӧ���Ʒ�Ӧ�¶���315~400��֮�䣬��Ӧ�¶ȹ��ͻ�Ӱ�췴Ӧ���ʣ����¶�Ҳ���˹��ߣ�ԭ����___________________��
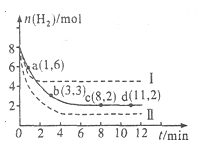
�۰�����[n(NH3)��n(NO)]��ֱ��Ӱ��÷����������ʡ�350��ʱ��ֻ�ı䰱����Ͷ��������Ӧ��x��ת�����백���ȵĹ�ϵ����ͼ��ʾ����X��________________ (�ѧʽ)����n(NH3)��n(NO)��1.0ʱ��������NOŨ�ȷ���������Ҫԭ����________________ ��

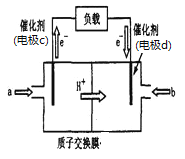
��3��ͨ��NOx�������ɼ��NOx�ĺ������乤��ԭ��ʾ��ͼ���£�

��Pt�缫�Ϸ�������______________��Ӧ�����������ԭ������
��д��NiO�缫�ĵ缫��Ӧʽ��___________________________________��
���𰸡���1��NO+CO![]() N2+CO2��2����-1265.5kJ/mol
N2+CO2��2����-1265.5kJ/mol
���¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ�ʹ��������Ҫ��Ӧ��ƽ�����淽���ƶ��������ʽ��ͣ�
��NH3 ����������������Ӧ����NO
��3���ٻ�ԭ��Ӧ ��NO��O2����2e��=NO2
��������
�����������1���������⣬NO��CO��ԭ��N2����Ӧ�Ļ�ѧ����ʽΪNO+CO![]() N2+CO2��
N2+CO2��
��2������֪��4NH3(g)+5O2(g)![]() 4NO(g)+6H2O(g)��H=-905.5kJmol-1�١�N2(g)+O2(g)
4NO(g)+6H2O(g)��H=-905.5kJmol-1�١�N2(g)+O2(g)![]() 2NO(g) ��H=+180kJmol-1�ڣ����ݸ�˹���ɢ�-�ڡ�2�ɵ�4NH3(g)+4NO(g)+O2(g)
2NO(g) ��H=+180kJmol-1�ڣ����ݸ�˹���ɢ�-�ڡ�2�ɵ�4NH3(g)+4NO(g)+O2(g)![]() 4N2(g)+6H2O(g)��H=��-905.5kJmol-1��-��+180kJmol-1����2=-1265.5kJ/mol��
4N2(g)+6H2O(g)��H=��-905.5kJmol-1��-��+180kJmol-1����2=-1265.5kJ/mol��
�ڸ÷���Ӧ���Ʒ�Ӧ�¶���315��400��֮�䣬��Ӧ�¶Ȳ��˹���Ҳ���˹��ߣ��¶�Խ�߷�Ӧ����Խ�죬�¶ȹ��ͣ���Ӧ����С���¶ȹ��ߣ�ʹ��������Ҫ��Ӧ��ƽ�����淽���ƶ��������ʽ��͡�
�۰�����[n(NH3)��n(NO)]Խ�����������ʵ���Խ������ת����ԽС����ͼ��֪�����Ű�����Ũ�ȵ�����X��ת���ʼ�С������XΪNH3����[n(NH3)��n(NO)]��1.0ʱ��������������Ӧ����NO������������NOŨ������
��3����ͼʾ��֪����ԭ��ط�ӦΪ2NO��O2=2NO2��NOΪ��ԭ����O2Ϊ��������O2��Pt�缫�õ��ӷ�����ԭ��Ӧ���缫��ӦΪO2��4e��=2O2����NO��NiO�缫��ʧ���ӷ���������Ӧ���缫��ӦΪNO��O2����2e��=NO2��