��Ŀ����
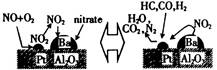
��15�֣����Ų��Ͽ�ѧ�ķ�չ�����������仯����õ���Խ��Խ�㷺��Ӧ�ã�������Ϊ���Ͻ��ά���ء���Ϊ�������ú�������������V2O5��VOSO4�������Բ�������������Ա����������һ�����ӽ��������շ����¹��գ������ʴ�91.7%���ϡ�
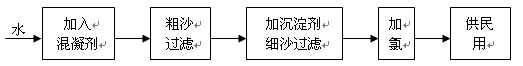
���ֺ���������ˮ�е��ܽ������±���ʾ��

�ù��յ���Ҫ�������£�

��ش��������⡣
�Ź�ҵ����V2O5ұ���������������ȼ������û�ѧ����ʽ��ʾΪ ��
�Ʒ�Ӧ�ٵ�Ŀ���� ��
�Ǹù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽ�� ��
������֪Ũ�ȵ������ữ��H2C2O4��Һ���ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к�������VO2++H2C2O4+H+��VO2++CO2+X��
XΪ ��д��ѧʽ����

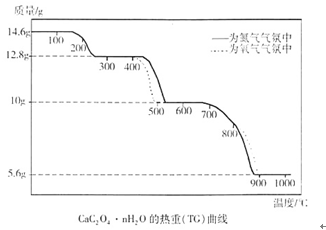
�ɾ������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯����������ͼ��ʾ����NH4VO3�ڷֽ������ ������ţ���
���ֺ���������ˮ�е��ܽ������±���ʾ��

�ù��յ���Ҫ�������£�

��ش��������⡣
�Ź�ҵ����V2O5ұ���������������ȼ������û�ѧ����ʽ��ʾΪ ��
�Ʒ�Ӧ�ٵ�Ŀ���� ��
�Ǹù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ��д���ò�������Ӧ�����ӷ���ʽ�� ��
������֪Ũ�ȵ������ữ��H2C2O4��Һ���ζ���VO2��2SO4��Һ���Բⶨ��Ӧ�ں���Һ�к�������VO2++H2C2O4+H+��VO2++CO2+X��
XΪ ��д��ѧʽ����

�ɾ������ط�����ã�NH4VO3�ڱ��չ����У����������ļ���ֵ�������꣩���¶ȱ仯����������ͼ��ʾ����NH4VO3�ڷֽ������ ������ţ���
| A���ȷֽ�ʧȥH2O���ٷֽ�ʧȥNH3 | B���ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O |
| C��ͬʱ�ֽ�ʧȥH2O��NH3 | D��ͬʱ�ֽ�ʧȥH2��N2��H2O |
��3V2O5��10Al6V��5Al2O3 �ƽ�V2O5ת��Ϊ�����Ե�VOSO4
��NH��VO===NH4VO3�� ��H2O��3�֣� ��B
��NH��VO===NH4VO3�� ��H2O��3�֣� ��B
��1����ҵ����V2O5ұ���������������ȼ������仯ѧ����ʽ��ʾΪ��
3V2O5��10Al6V��5Al2O3��
��2��������ͼ��֪��Ӧ�ٵ�Ŀ���ǣ���V2O5ת��Ϊ�����Ե�VOSO4��
��3���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ����Ӧ�����ӷ���ʽΪ��
NH��VO===NH4VO3����
��4���������غ㶨�ɿ�֪XΪ��H2O��
��5����ͼ�����ɵã�NH4VO3�ڷֽ�������ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O��
3V2O5��10Al6V��5Al2O3��
��2��������ͼ��֪��Ӧ�ٵ�Ŀ���ǣ���V2O5ת��Ϊ�����Ե�VOSO4��
��3���ù����з�Ӧ�۵ij����ʣ��ֳƳ����ʣ��ǻ��շ��Ĺؼ�֮һ����Ӧ�����ӷ���ʽΪ��
NH��VO===NH4VO3����
��4���������غ㶨�ɿ�֪XΪ��H2O��
��5����ͼ�����ɵã�NH4VO3�ڷֽ�������ȷֽ�ʧȥNH3���ٷֽ�ʧȥH2O��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 HCl+HClO K=4.5��10-4
HCl+HClO K=4.5��10-4